FDA announces another recall for surgical devices over safety concerns—7 new injuries reported
Maquet Cardiovascular, a subsidiary of Getinge, has issued an additional recall for its endoscopic vessel harvesting (EVH) devices over significant safety concerns, according to the U.S. Food and Drug Administration (FDA).
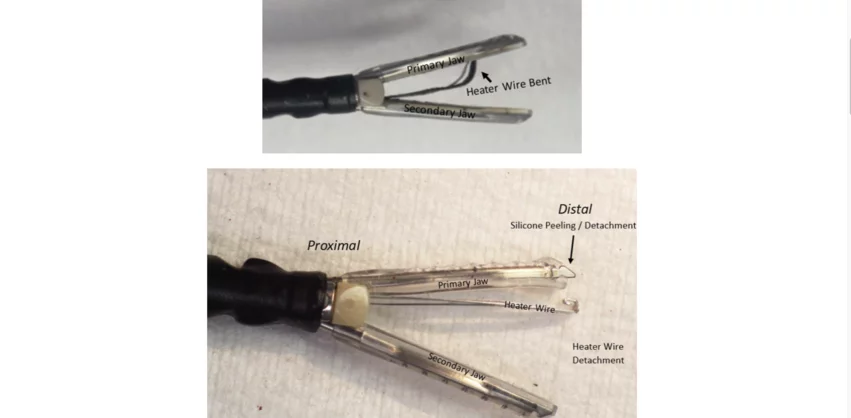
This notice covers all unexpired lots of Maquet’s VasoView HemoPro 2 Endoscopic Vessel Harvesting System and VasoView HemoPro 2 (w/VasoShield) Endoscopic Vessel Harvesting System. The recall was put into place due to ongoing issues with heater wires becoming bent or detached and silicone peeling or detaching from the device. Seven serious injuries have been linked to these two problems—four due to the heater wire issue and three due to the silicone peeling or detaching. No patient deaths have been reported.
These devices do not have to be returned, but new instructions have been provided to reduce the risk of further issues.
This is a Class I recall, which means the FDA believes the devices “may cause serious injury or death” if used without following the recall notice’s instructions.
Customers advised to read instructions, inspect devices closely to minimize risks
The new advisory urges all customers to pay close attention to the instructions for use (IFU) of these devices.
In addition, certain actions are recommended that can help mitigate the risk of any issues:
- Inspect the devices prior to use for any signs of damage, including silicone that may be peeling away from the jaws.
- Check the devices for rough surfaces, sharp edges and any “unusual protrusions” that could be considered hazardous.
- Monitor the devices during use for any signs of silicone peeling away.
- Inspect the devices after use to ensure no parts are missing or damaged.
- Stop using the device if any parts suddenly become damaged or missing.
- “Locate and remove” any parts of the devices that may break off during use.
- Monitor patients for complications such as the delayed onset of pain, infection or allergic reactions.
Issues continue for Maquet Cardiovascular’s endoscopic vessel harvesting devices
Back in November 2024, the company recalled some of its VasoView HemoPro 1.5 and VasoView HemoPro 1 EVH devices after receiving multiple reports of silicone parts breaking off during the harvesting process. Seventeen serious injuries had been reported at the time, and the FDA recommended that all impacted devices be returned immediately.
When that initial recall was announced, the FDA indicated it was examining additional issues. Those issues appear to be the ones covered in this latest announcement.