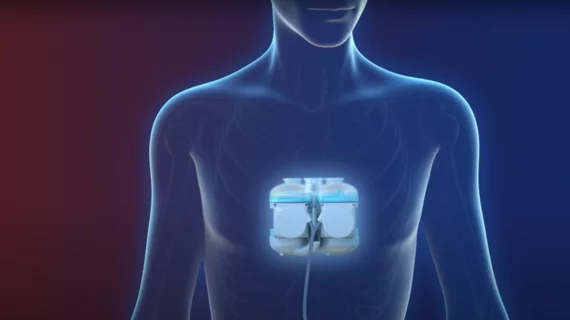
Swedish total artificial heart tech granted FDA’s humanitarian use designation
Scandinavian Real Heart AB (Realheart), a Swedish medtech company, has received the FDA’s humanitarian use device (HUD) designation for its total artificial heart (TAH) technology. This means certain U.S. patients with advanced biventricular heart failure could potentially receive the Realheart TAH as they wait for a heart transplant.
The FDA’s HUD designation is granted to medical devices designed to treat specific diseases or conditions in patients with limited other options. Companies apply for HUD stats by submitting outcomes data and other documentation to the agency, explaining how the device works and why it is needed.
“We are extremely pleased that Realheart TAH has been granted HUD designation by the FDA,” Laura Perkins, CEO of Realheart, said in a statement. “It is gratifying to look forward to our efforts culminating in such humanitarian benefit. We look forward to updating the market on further clinical and regulatory activities and meanwhile continue our preclinical development,which will be important for a potential future full market approval.”
Preclinical studies focused on the Realheart TAH’s safety and effectiveness are currently underway. The company hopes these will pave the way for clinical studies of heart failure patients, which could then help the TAH gain full approval from the FDA and other regulatory agencies. In November, Realheart shared early data from some of those preclinical studies, noting that the TAH technology performed well in patients during sleep and exercise.
“This is very promising data as it is likely that patients will have a better quality of life if the artificial heart can automatically adapt to the body's activity level,” Perkins said at the time.