Abiomed heart pump gains FDA approval to treat acute right heart failure
Abiomed’s Impella RP Flex with SmartAssist heart pump has received approval from the U.S. Food and Drug Administration (FDA) for the treatment of acute right heart failure for up to 14 days.
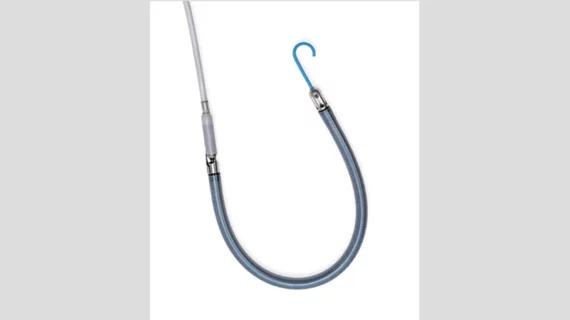
The heart pump, which is implanted via the patient’s internal jugular vein, includes dual-sensor technology that helps clinicians track data and manage different settings as needed. The device also includes an 11 French indwelling catheter, designed to enable easy mobility, and a flexible cannula.
Abiomed also emphasized that its Impella RP platform of devices include built-in mechanical circulatory support technology and do not require extracorporeal blood circulation.
“Impella RP Flex demonstrates Abiomed’s ongoing commitment to improving patient survival and achieving native heart recovery,” cardiothoracic surgeon Mark B. Anderson, MD, chairman of the department of cardiac surgery for the Heart and Vascular Hospital at Hackensack University Medical Center, said in a prepared statement.
“The complexity of right ventricular failure has resulted in patients being underdiagnosed and undertreated,” added interventional cardiologist Robert Salazar, MD, director of cardiovascular research at Kingwood Medical Center. “Impella RP Flex is a novel tool that gives physicians the flexibility to treat this challenging patient population.”
The new FDA indication reads as follows:
“The Impella RP Flex with SmartAssist System is indicated for providing temporary right ventricular support for up to 14 days in patients with a body surface area ≥1.5 m2, who develop acute right heart failure or decompensation following left ventricular assist device implantation, myocardial infarction, heart transplant, or open-heart surgery.”
According to Abiomed, the newly approved device will be rolled out in the United States sometime this quarter.