Cardiologists make case for increasing use of device-based therapies for heart failure
Advanced device-based therapies are associated with significant benefits for heart failure patients and should be used alongside traditional pharmaceutical treatments, according to a new scientific statement from the Heart Failure Society of America (HFSA).
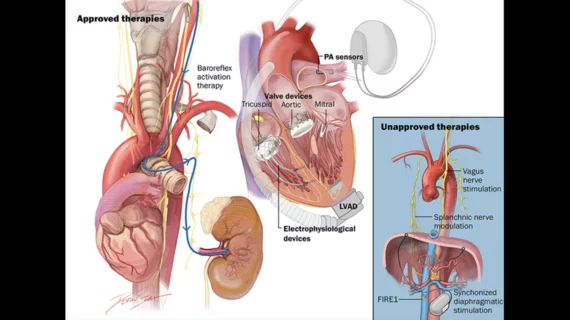
The statement, published in full in the Journal of Cardiac Failure, examines a number of new-look medical devices that have emerged in recent years as additional ways to treat heart failure.[1] Implantable cardioverter-defibrillators (ICDs) and cardiac resynchronization therapy (CRT) already play a prominent role in guideline-directed medical therapy (GDMT), but newer therapies such as baroreflex activation therapy (BAT), cardiac contractility modulation and established structural heart techniques such as transcatheter aortic valve replacement and mitral valve edge-to-edge repair can also provide considerable value for heart failure patients who do not respond to other treatments.
The statement’s authors advocate for a new pathway that begins with three to six months of traditional GDMT and then expands to include device-based therapies when appropriate. The group also highlights the possibility that safe and effective heart failure treatments are being underused by modern healthcare providers; a personalized approach that considers an individual patient’s symptoms and overall health is recommended.
“Our consensus document defines the unmet need in chronic heart failure and the important role of device-based therapies in bridging heart failure gaps,” co-lead author Jerry D. Estep, MD, division chair of cardiovascular medicine with Cleveland Clinic Florida, said in a statement. “We provide detailed information on the different categories of heart failure devices and use considerations based on associated contemporary outcome data. We hope our document will improve patient outcomes by providing a clinical framework to guide implementation of current and future FDA approved device-based therapies.”
Click here to read the full scientific statement in the Journal of Cardiac Failure.
Register now to learn more about baroreflex activation therapy
BAT with the FDA-approved Barostim device from CVRx is one of many device-based therapies detailed in the HFSA scientific statement. Barostim works by sending small electrical pulses to certain baroreceptors in the neck, which then send signals to the brain to help regulate the patient’s heart, kidneys and vascular system. These signals are typically sent without therapy, but this function is diminished in heart failure patients, leading to significant health risks.
Cardiovascular Business is hosting a free webinar focused on the Barostim device on Thursday, Oct. 24. The webinar will include an expert panel and a live Q&A session. Topics are expected to include how the device works, billing and reimbursement, prior authorization policies and how to increase awareness about the device among referring physicians.
Click here for more information about the upcoming webinar, including how to register.