CMS increases inpatient payment for implantable Barostim device
CVRx, a Minneapolis-based medical device company focused on heart failure treatments, announced that the Barostim implant procedure is now associated with a higher payment in the 2025 Medicare Hospital Inpatient Prospective Payment System (IPPS) final rule.
The U.S. Centers for Medicare and Medicaid Services (CMS) published the final 2025 IPPS on Aug. 1. Implanting the Barostim device was previously assigned to MS-DRGs 252, 253 and 254, and its average payments ranged from $17,000 to $23,000. Now, however, the device has been reassigned to MS-DRG 276, which has an average payment of approximately $43,000. The updated payment officially takes effect on Oct. 1.
“We applaud CMS’ consideration and recognition of the resource requirements associated with the Barostim implant procedure,” Kevin Hykes, president and CEO of CVRx, said in a statement. “We believe that reassignment to MS-DRG 276 will facilitate increased access to the therapy for patients with heart failure by establishing sufficient reimbursement for the procedure when performed in an inpatient setting.”
A previous update from CMS
In November 2023, CMS included a higher reimbursement for the Barostim device in its 2024 Medicare Hospital Outpatient Prospective Payment System (OPPS). While the implant had previously been associated with an average outpatient payment of $29,000, the updated OPPS increased that average payment to $45,000.
What is the Barostim implant?
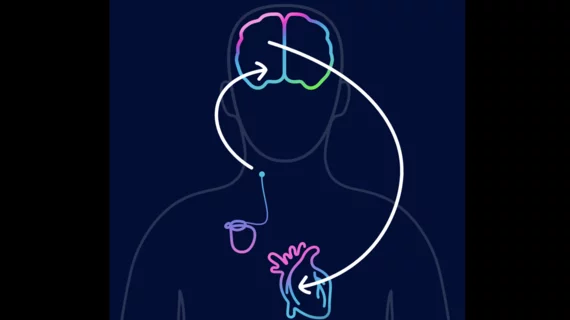
Barostim is an implantable device designed for the treatment of patients with congestive heart failure and a low or reduced ejection fraction. It has gained both U.S. Food and Drug Administration and CE mark approval.
The implant works by sending small electrical pulses to certain sensors in the neck, which then send signals to the brain to help regulate the patient’s heart, kidneys and vascular system. These signals are typically sent without therapy, but this function is diminished in heart failure patients, leading to significant health risks.
Patients who already have a defibrillator are still eligible to receive a Barostim device. In fact, a majority of Barostim patients already have a defibrillator.