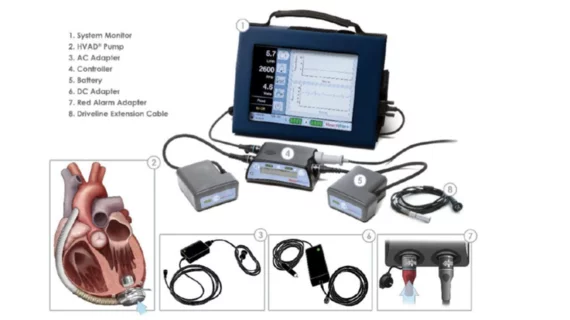
FDA announces another recall of Medtronic HVAD pump implant kits—1 patient death reported
The U.S. Food and Drug Administration (FDA) has announced that Medtronic is once again recalling its HeartWare Ventricular Assist Device (HVAD) pump implant kit, a part of the company’s HeartWare HVAD system. This is a Class I recall, which means using the device can lead to serious injury or death. The new recall includes more than 1,600 devices distributed from October 2006 to June 2021.
The reason for this recall is that Medtronic has identified a defect in the pump implant kit’s construction.
“Following an inspection of explanted pumps returned to Medtronic, an analysis showed moisture had entered the center post of the pump causing corrosion and demagnetization of the internal magnets, which may cause the pump to rotate incorrectly,” according to an advisory on the FDA’s website. “Patients with affected devices may present with signs and symptoms that resemble pump thrombosis.”
The defect is associated with a risk of “pump malfunction, death or severe injury.” Patients may also have to undergo surgery and have the pump replaced.
There have been three complaints related to this issue; one patient died and two others were injured.
Prior issues with Medtronic’s HVAD pump implant kits, HVAD systems
Back in March 2021, Medtronic had to recall its HVAD pump implant kits after receiving numerous complaints that the devices “may fail to initially start, restart or have a delay in restarting after the pump was stopped.” There were 29 complaints at the time of that recall, including two deaths and 19 serious injuries.
That recall included kits labeled as PUMP 1103, PUMP 1104 or PUMP 1104JP. This latest recall included those same models as well as two others: 1101 and MCS1705PU.
In June 2021, Medtronic stopped the sales and distribution of its HVAD system due to multiple issues, including the ongoing problems with its pump implant kits.
“The FDA has monitored the performance of the Medtronic HVAD System since it was approved in 2012, including monitoring neurological adverse event rates,” the agency said at the time. “Although Medtronic has stopped the sale and distribution of new HVAD Systems, the FDA will continue to monitor the safety and effectiveness of the HVAD systems that remain implanted.”
“There is nothing more important than the safety and well-being of patients,” Nnamdi Njoku, president of Medtronic’s Mechanical Circulatory Support Business, said in a separate statement at the time. “We recognize this information may be concerning for patients and their caregivers, and Medtronic is committed to supporting them in coordination with their physicians.”
The story did not end there; Congress announced an investigation into how the FDA handled issues with the HVAD system in March 2022.
“I am concerned by FDA’s slow action, over multiple administrations, to protect patients from this product despite early warning signs,” Rep. Raja Krishnamoorthi said in an open letter.
Related FDA Recall Content
FDA announces Class I recall of 4,800 imaging catheters due to risk of vascular injury
FDA announces another recall of common hypertension medication due to potential cancer risk