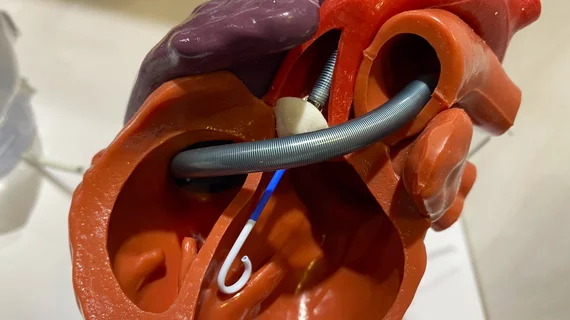
FDA approves J&J MedTech’s Impella heart pumps to treat pediatric patients
Johnson & Johnson MedTech has announced that its Impella 5.5 with SmartAssist and Impella CP with SmartAssist heart pumps are now approved by the U.S. Food and Drug Administration (FDA) to treat pediatric patients with symptomatic acute decompensated heart failure and cardiogenic shock. The two devices have already been approved by the FDA for the treatment of adult patients for a number of years.
“The opportunity to treat the hearts of pediatric patients with our life-supporting technology is incredible and fills us with gratitude,” Sonya Bhavsar, PhD, senior director of research and development for Johnson & Johnson MedTech’s ECP & pediatrics platform, said in a statement. “This milestone motivates us to continue innovating solutions to increase the number of life years that these patients have and can spend with their families and loved ones.”
Johnson & Johnson MedTech partnered with the Advanced Cardiac Therapies Improving Outcomes Network (ACTION), known globally for its commitment to patient safety, to confirm these devices could safely and effectively treat younger patients. The groups plan on developing training programs specifically designed to help clinicians provide the best care possible when treating pediatric patients with these heart pumps.
“This marks a monumental achievement for children with heart failure as, historically, this area of pediatric care has been underfunded and understudied,” Angela Lorts, MD, MBA, and David Rosenthal, MD, co-founders of ACTION. “We are proud to have worked with Johnson & Johnson MedTech on this crucial approval and look forward to further collaborations that will enhance care for these vulnerable patients.”
The Impella heart pumps were marketed and sold by Abiomed for many years before Johnson & Johnson acquired the company for $16.6 billion in 2022. As of September, Abiomed now exclusively goes by the name Johnson & Johnson MedTech.