FDA approves new pulmonary artery sensor for heart failure
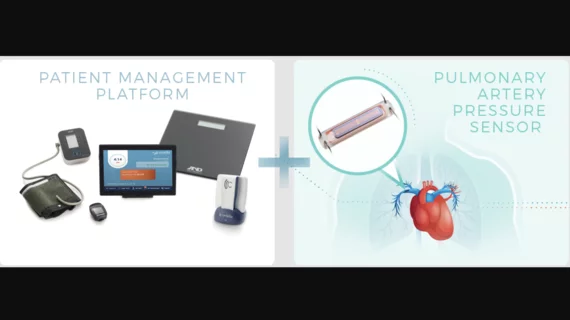
Endotronix, an Illinois-based healthcare technology company, has gained U.S. Food and Drug Administration approval for its Cordella Pulmonary Artery (PA) Sensor System, which uses PA pressure-guided therapy to manage and treat heart failure patients.
The Cordella platform includes an implantable sensor that tracks the patient’s PA pressure and other helpful health metrics, including blood pressure, pulse, weight and various symptoms. Those data are then used to manage care and make treatment decisions as needed. PA pressure is known as an early indicator of congestion—and can be used to determine when patients may need additional care.
The FDA’s decision was based largely on data from the PROACTIVE-HF pivotal trial, which examined data from patients with New York Heart Failure (NYHA) class III heart failure. Back in October 2023, researchers presented new 12-month data from the study at the Heart Failure Society of America’s annual meeting in Cleveland.
“This approval is very exciting and has the potential to transform care for heart failure patients,” PROACTIVE-HF principal investigator Liviu Klein, MD, MS, section chief of advanced heart failure, mechanical circulatory support, pulmonary hypertension and heart transplant at the University of California San Francisco, said in a statement. “Endotronix's solution provides a more complete clinical picture of the patient, so providers are able to make informed remote care decisions between office visits. PROACTIVE-HF demonstrated that with Cordella clinicians achieved more optimal and timely dosing of key HF medications, significantly improving outcomes. In addition, the easy-to-use platform engages patients to drive consistent daily habits and self-awareness of trends to support sustainable lifestyle changes.”
Endotronix expects to officially launch the Cordella platform by the end of 2024. The company has already started work on the PROACTIVE-HF 2 study and expect to enroll up to 1,500 patients with NYHA class II heart failure from the United States and Europe.