FDA clears AI model for detecting signs of heart failure in ECGs
Anumana, a Massachusetts-based nference portfolio company, has received U.S. Food and Drug Administration (FDA) clearance for its new artificial intelligence (AI) model that identifies signs of low ejection fraction (LEF) in 12-lead electrocardiograms (ECGs).
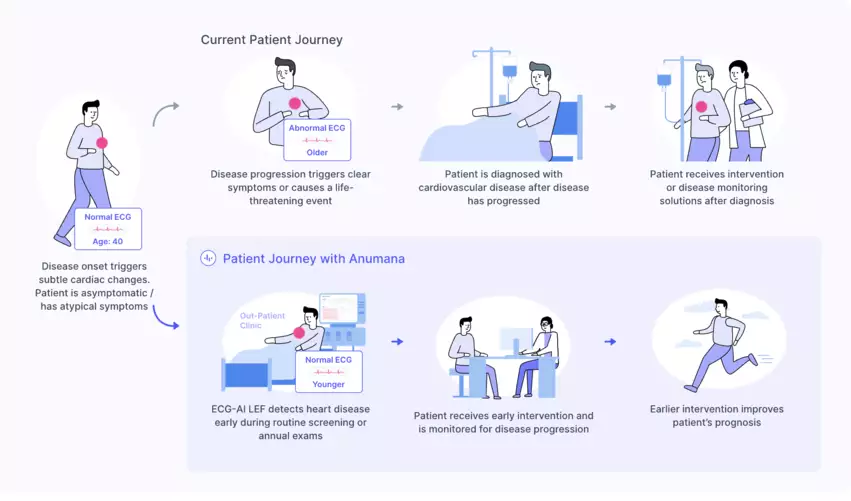
The ECG-AI LEF algorithm, developed as part of a collaboration with Mayo Clinic in Rochester, Minnesota, was designed to help screen high-risk patients for heart failure. It was built using data from more than 100,000 ECGs and 100,000 echocardiograms and has already been tested in a clinical setting on more than 40,000 patients. The AI model was then validated in a clinical trial involving 16,000 patients, producing a sensitivity of 84.5% and specificity of 83.6% when it came to spotting patients with an ejection fraction less than or equal to 40%.
“Anumana’s ECG-AI LEF fills an important unmet need—the lack of an easily accessible point-of-care, noninvasive, and inexpensive tool to screen for a weak heart pump,” Paul Friedman, MD, chair of the department of cardiovascular medicine at Mayo Clinic and chair of the Anumana Board of Advisors, said in a prepared statement. “It allows identification of otherwise hidden disease, for which many effective, lifesaving treatments are available—once the presence of the disease is known.”
“Anumana was established in 2021 by nference in partnership with Mayo Clinic to unlock the electrical language of the heart through deep learning and improve disease diagnosis and patient care,” added Murali Aravamudan, co-founder and CEO of Anumana and nference. “In the short time of two years we have secured multiple FDA breakthrough device designations, entered multi-year agreements with three pharma partners, successfully established two new medical procedure codes for ECG AI technology, and now achieved our first FDA breakthrough medical device clearance. This is a significant milestone, and we are excited about the next phase of the journey, deploying our technology in the U.S. and globally to empower clinicians and enhance real-world clinical care.”
Other Anumana algorithms “read” ECG results in search of signs of cardiac amyloidosis, pulmonary hypertension and hyperkalemia.