Former FDA employees share concerns about heart device safety data
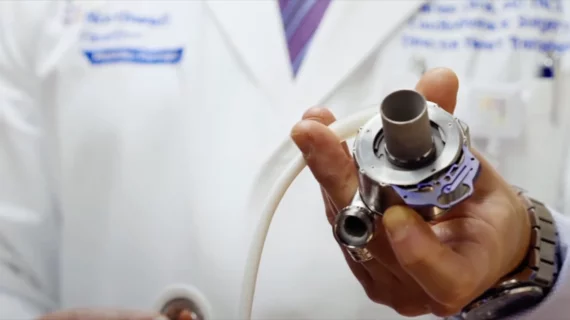
Abbott’s HeartMate 3 left ventricular assist device (LVAD), a heart pump approved by the U.S. Food and Drug Administration (FDA) for the long- and short-term treatment of patients with advanced heart failure, has been implanted in thousands of U.S. patients in recent years. It is the only device of its kind currently available in the United States for an especially vulnerable patient population.
According to a new investigative report from CBS News and KFF Health News, however, safety data related to the device have raised questions in certain parts of the healthcare industry. For example, according to the report, more than 4,500 safety incidents about the Abbott heart pump have been filed since August 2017. Thoratec, a subsidiary of Abbott, reviewed these reports, finding that there was no problem with the device or how the device was used nearly 90% of the time.
CBS News and KFF Health News spoke with industry veterans, Abbott representatives and even heart patients currently using the HeartMate 3. Two former FDA employees were among the people interviewed, and both shared concerns about the high number of adverse events that have been linked to the device.
“To me this is a safety signal and it's hard to know if the FDA is working on something to address it," said Madris Kinard, a former FDA employee who went on to found Device Events, a company that works to make FDA data easier to understand for the public. “You have to wonder why [death reports are] still happening, and at the same rate.”
“The FDA may be missing some signals,” added Larry Kessler, a former director of the FDA’s Office of Surveillance and Biometrics.
Meanwhile, a current FDA spokesperson emphasized that the agency still fully supports the device as a treatment option for high-risk heart failure patients.
The only device of its kind on the market
HeartMate 3 is the only FDA-approved device of its kind currently available on the U.S. market. Medtronic did sell its own ventricular assist device, HeartWare, but stopped selling and distributing it to patients back in 2021 due to evidence that its use may put patients at a serious risk of death or other adverse outcomes.
There is no evidence that Abbott would need to consider taking similar actions.
While Abbott representatives declined to speak the investigative report’s authors about specific adverse events, public affairs director Justin Paquette did provide a statement on the topic to Cardiovascular Business.
“The HeartMate 3 heart pump is a critically important option for people battling late-stage heart failure who are often extremely sick and out of therapy options, and the pump has set the standard for clinical outcomes associated with LVAD therapy," he said.
That is just the beginning of the information available in this this investigative piece. Click the link below to read the full report from CBS News and KFF Health News: