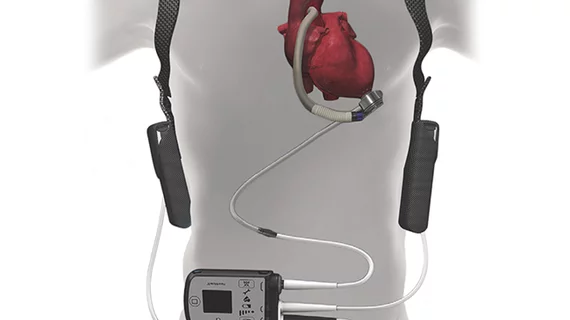
HeartMate 3 heart pump gets FDA approval as destination therapy
The HeartMate 3 left ventricular assist device (LVAD) has gained FDA approval for advanced heart failure patients ineligible for a transplant, manufacturer Abbott announced Oct. 19.
The heart pump was approved as a bridge-to-transplant option in August 2017, but is now available for use as a destination therapy. It has been approved in Europe for both indications since October 2015.
Results of the MOMENTUM 3 study, which was published in March in the New England Journal of Medicine, supported the expanded indication, according to Abbott. That trial showed an 82.8 percent survival rate at two years for patients who had New York Heart Association Class IIIB or Class IV heart failure. Rates of suspected pump thrombosis and stroke at two years were 1.1 percent and 10 percent, respectively, for the continuous-flow LVAD.
"Approximately a quarter of a million people (in the U.S.) are living with advanced heart failure, and many of these people will need a heart transplant; however, only a few thousand will receive a new heart," Nir Uriel, MD, the director of Heart Failure, Transplant and Mechanical Circulatory Support at the University of Chicago School of Medicine, said in a press release.
"The destination therapy approval for Abbott's HeartMate 3 device now gives these patients new hope that they can receive a heart pump clinically proven to mitigate challenges we've historically confronted with this therapy—stroke and blood clotting—while also offering survival rates on par with transplant."
One of the device changes in the HeartMate 3 versus the HeartMate 2 was using a magnetically levitated pump, which was engineered “to reduce shear stress on blood elements and avert pump thrombosis,” according to the NEJM study.