Researchers share first human data on new interventional shunt procedure for HFpEF
Percutaneous radiofrequency ablation–based interatrial shunting could be a safe and effective treatment option for patients with heart failure with preserved ejection fraction (HFpEF), according to new first-in-man study published in Circulation: Heart Failure.[1]
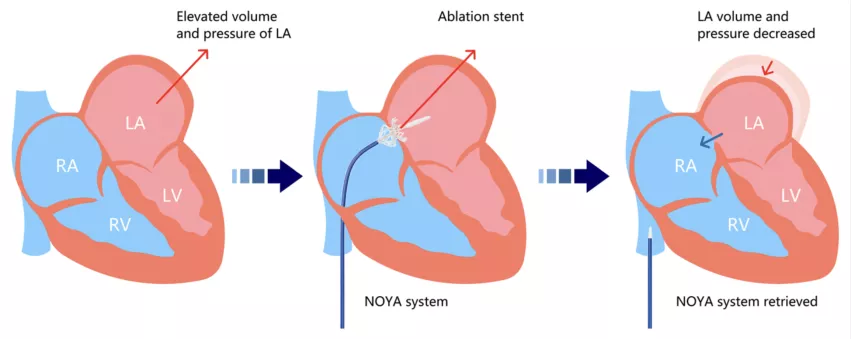
The study’s authors noted that significant progress has been made in the treatment of heart failure with reduced ejection fraction (HFrEF), but clinicians still struggle with the high mortality and morbidity associated with HFpEF. Seeing the success other researchers were having reducing left atrial pressure (LAP) with artificial atrial septal defects, the group aimed to see if a similar strategy could successfully be used to treat HFpEF with the RAISE trial.
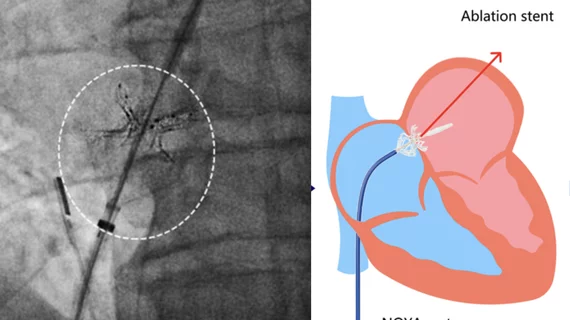
The group enrolled 10 HFpEF patients treated from November 2018 to November 2019. The mean age was 60 years old, and 60% of patients were women. A novel adjustable shunt device with adjustable waist diameter — the NoYA system, developed by NoYA MedTech — was used for all procedures. The device was removed from the body once each procedure was complete, leaving an artificial atrial septal defect behind. Intracardiac echocardiology guidance was used for 60% of patients, transesophageal echocardiography was used for the remaining 40%. The mean procedure time was 90 minutes.
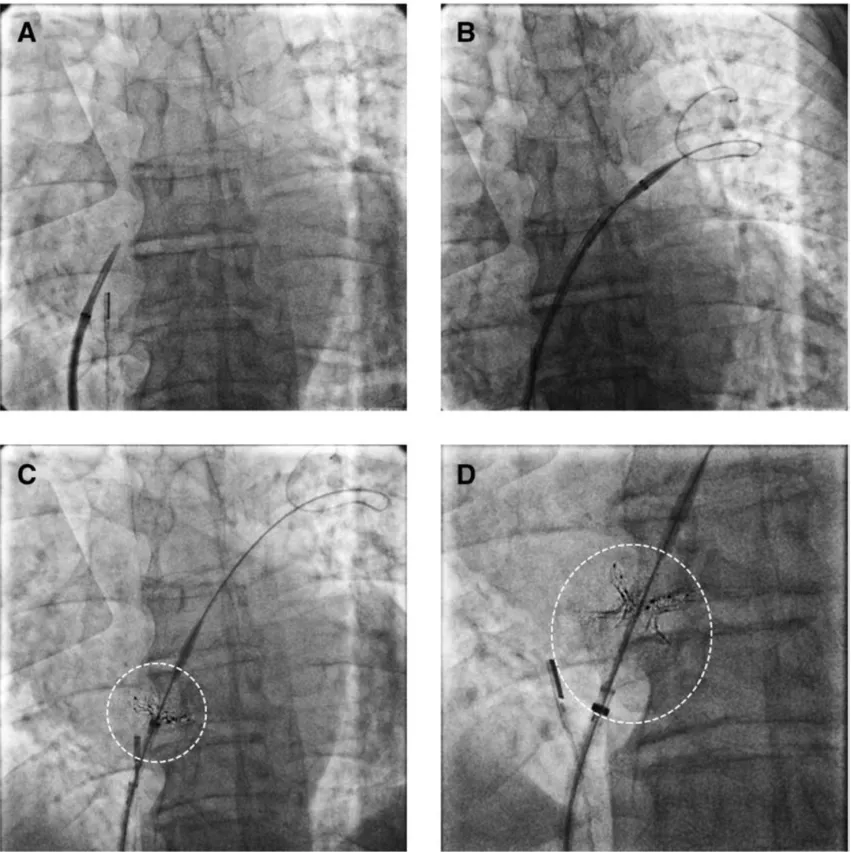
Fluoroscopy images of the first-in-man study of a new intra-atrial septal shunt to lower pressures between the upper chambers of the heart in heart failure patients. The series shows the positioning of an RF ablation catheter to cut a hole between the chambers and deployment of a stent structure to enlarge the hole. Image courtesy of Sun et al. and Circulation: Heart Failure.
Overall, the researchers found, treatment with a radiofrequency ablation-based interatrial shunt (RAIAS) was a success. The procedure success rate was 100%, and no major safety events — including thromboembolism — were reported. Six-month follow-up was completed for all patients except one, and that was due to complications related to the COVID-19 pandemic. Reductions in LAP were seen 30 minutes following the procedure. The mean diameter of the interatrial defect was 5 mm immediately after the procedure and 3 mm after six months.
While NT-proBNP levels were consistently reduced among these patients, six-minute walk distances consistently improved. Eight patients saw improvements in their New York Heart Association class.
“Generally, the preliminary data suggested that the RAIAS therapy was a safe and feasible strategy for patients with HFpEF (or heart failure with mid-range ejection fraction) and the therapy was easy to operate and learn,” wrote lead author Dr. Wei Sun, a cardiologist with the First Affiliated Hospital of Nanjing Medical University in China, and colleagues. “Future prospective randomized clinical trials are needed to clarify the efficacy and long-term safety.”
Device similar to the Corvia heart failure shunt
This device technology is similar to the Corvia Atrial Shunt, which had data presented at the Technology and Heart Failure Therapeutics (THT) 2022 conference. There were high hopes that device would offer significant relief of heart failure symptoms, but the data at THT in February did not show this in all patients. Results showed little difference with sham procedures. However, the study did identify there may be a specific responder group to this therapy that merited further research.
Read more on the Covia THT data in the story Implantable atrial shunt therapy trial identifies treatable HFpEF patients.
Related Heart Failure Content:
Cholesterol medications, flu shots and heart failure: Day 2 at ACC.22
Cardiology groups debut new heart failure guidelines ahead of ACC.22
Congress investigating FDA over heart device recall, says agency ‘failed to protect consumers’
COVID-19 vaccines safe for patients with a history of heart damage
Reference: