Epicardial ablation in Brugada syndrome reduces sudden cardiac death
A late-breaking study found epicardial ablation in Brugada syndrome (BrS) helped significantly reduce sudden cardiac arrest in these patients, increasing their survival. The results of the Epicardial Ablation in Brugada Syndrome to Prevent Sudden Death trial was presented at Heart Rhythm 2022, the annual meeting of the Heart Rhythm Society (HRS).
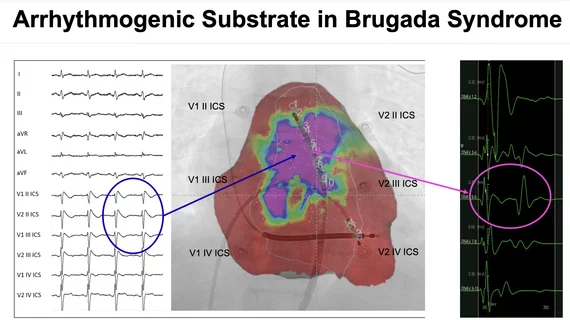
There have been earlier studies that deified arrhythmogenic substrate in the myocardium in Brugada syndromes that appears to be the cause of the arrhythmia that trigger sudden cardiac death. While this has been treated using implantable cardioverter defibrillators (ICD), there is still a high number of Brugada patients who die.
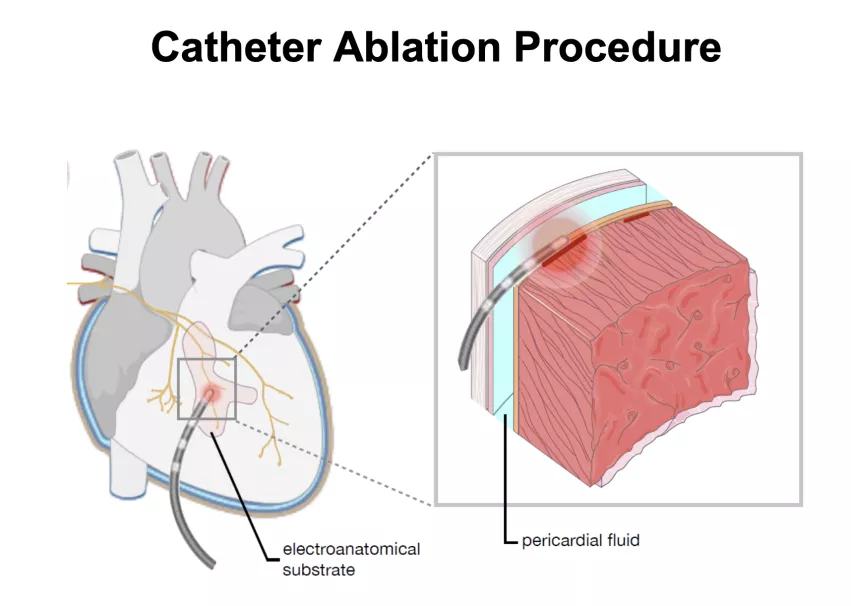
The study, started in 2017, was designed to show catheter ablation is a safe procedure available for BrS patients at risk of sudden death.
"The discovery of the ‘Brugada substrate’ opens avenues enabling further therapeutic strategies," explained Giuseppe Ciconte, MD, PhD, Department of Arrhythmia and Electrophysiology, IRCCS Policlinico San Donato, Milano, Italy, who presented the data. "Epicardial electrical abnormalities represent the hallmark phenotype signature of the disease. Arrhythmogenic substrate ablation successfully reduced the VT/VF burden, preventing recurrent ICD therapies."
A primary outcome of the study was freedom of ventricular fibrillation and pulseless ventricular tachycardia (VT/VF) recurrences that cause sudden cardiac arrest. There ablation. Group saw freedom from VT/VF at 95% at 36 months, versus 67% in the ICD group.
The aim of this study was to develop evidence-based treatment with optimal benefit for patients with BrS in terms of VT/VF burden reduction. Patients in the trial are randomized to epicardial catheter ablation of the arrhythmogenic substrate and ICD therapy (ablation arm) vs ICD therapy alone (control arm).
There were 150 patients enrolled and randomized to receive ablation or not in a 2:1 fashion (ablation plus ICD 105 patients vs. ICD only arm with 45 patients). All patients were then clinically followed up every 6 months for the ICD only group and after 1, 3 and 6 months, then every 6 months for the ablation and ICD group.
Ciconte showed a case of one patient where there was an abolition of abnormal potentials after RF and no further VF episodes after ablation.