FDA approves world’s first dual-chamber leadless pacemaker system
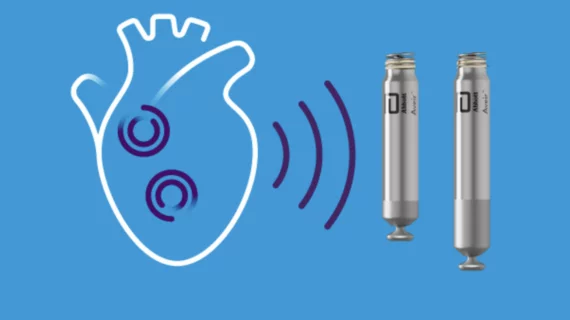
The U.S. Food and Drug Administration (FDA) has approved Abbott’s Aveir dual chamber (DR) leadless pacemaker system, the world’s very first dual-chamber leadless pacing solution for treating patients with abnormal heart rhythms. This is seen as a major advance in electrophysiology (EP) because it opens leadless pacing up to the majority of patients. While single-chamber leadless pacing has been around for several years, most bradycardia patients require dual-chamber pacing.
The minimally invasive system is made up of two Aveir pacemakers smaller than a AAA battery. One pacemaker, Abbott’s Aveir VR single chamber device, is placed on the patient’s right ventricle. The other pacemaker, the newly approved Aveir AR single chamber device, is placed on the patient’s right atrium. Both pacemakers are delivered using a catheter, and they communicate with each other using Abbott’s proprietary i2i technology, which uses high-frequency pulses to relay messages back and forth.
The system eliminates the need to create a surgical pocket for the pacemaker or transvenous leads, which EP experts say will reduce the number of complications and infections associated with pacemaker implants.
The regulatory approval is expected to open up leadless pacemaker technology to a significantly larger group of patients dealing with abnormal heart rhythms than ever before.
“Leadless pacemakers have been limited to a single chamber device because seamless, wireless synchronization of two pacemakers has been an insurmountable engineering challenge—until now,” Randel Woodgrift, senior vice president of Abbott's cardiac rhythm management business, said in a prepared statement. “Our team of dedicated scientists and engineers solved one of medtech's complex challenges in treating abnormal heart rhythms with the Aveir pacemaker, a tiny device packed with powerful technology.”
Late-breaking data support the FDA’s approval of the Aveir dual-chamber leadless pacemaker system
The FDA’s approval was based in part on late-breaking clinical data first presented at Heart Rhythm 2023, the Heart Rhythm Society’s 2023 annual meeting held in May. The Aveir DR i2i Study focused on data from 300 patients who presented with either sinus-node dysfunction or atrioventricular block. The implantation success rate was 98.3%, and the study’s primary safety endpoint was met in 90.3% of patients. The trial’s results were published in full in the New England Journal of Medicine.[1]
“We exceeded the pre-specified performance goals that were decided in advance with the regulatory authorities. In the electrical performance, we saw more than 95% achievement,” co-principal investigator Daniel Cantillon, MD, formerly from Cleveland Clinic and now senior vice president of global cardiovascular solutions for Masimo, told Cardiovascular Business back in May. “The communication between the upper and lower chambers to allow synchronous pacing achieved a 97% success rate. It showed this system is safe and effective and may provide a better solution to allow us to get the benefits of leadless cardiac pacing to a broader population of patients.”