Medtronic’s first-of-its-kind ICD meets safety endpoints in global clinical trial
Medtronic shared some good news with attendees at ESC Congress 2022 in Barcelona, the annual meeting of the European Society of Cardiology, noting that its Extravascular Implantable Cardioverter Defibrillator (EV ICD) system met its safety endpoints in a global clinical trial.
Findings from the EV ICD study were presented at ESC Congress 2022 Aug. 28 and simultaneously published in the New England Journal of Medicine.[1]
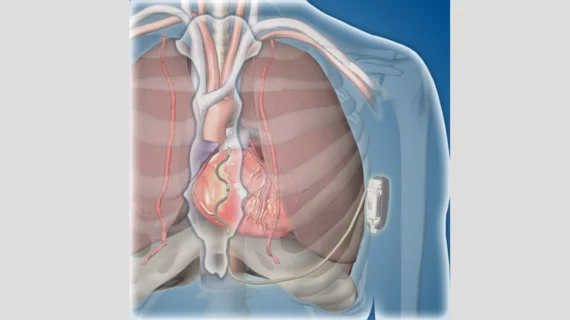
The lead of this new-look ICD is placed under the patient’s breastbone, outside of their heart and veins. This placement is intended to help clinicians avoid long-term complications such as vessel occlusion, blood infections or the need for intravenous lead extraction. The lead is then connected to a device implanted below the patient’s left armpit. The device is still investigational at this point; it has not been approved for sale or distribution.
Overall, researchers found that the EV ICD achieved a defibrillation success rate of 98.7%—the original goal was 88%—and met all safety endpoints. After six months, 7.3% of 316 patients had experienced a major complication.
Participants in the EV ICD study received “the same therapies provided by traditional ICDs,” according to Medtronic. This included defibrillation, anti-tachycardia pacing and back-up pacing therapies.
“We are very encouraged by the high defibrillation effectiveness and strong safety profile of the EV ICD system seen in the study, as we look to deliver less-invasive treatment options for patients at risk of sudden cardiac arrest,” co-author and ESC Congress 2022 presenter Ian Crozier, MBChB, MD, a cardiologist with Christchurch Hospital in New Zealand, said in a statement. “These results demonstrate the potential for this novel technology to be used as a safe, successful approach for patients with life-threatening arrhythmias.”
“These pivotal data mark the start of a new era in ICD therapy for patients who are at significant risk of dangerously fast heart rhythms,” added Alan Cheng, MD, chief medical officer of Medtronic’s cardiac management business.
The EV ICD is similar in placement and function to the Boston Scientific Subcutaneous Implantable Defibrillator (S-ICD) system, which gained regulatory approval s few years ago.