ACC, SCAI, HRS release consensus statement on left atrial appendage occlusion requirements
Three leading cardiology societies released a consensus statement on Dec. 10 regarding criteria institutions and operators should follow for left atrial appendage occlusion.
The recommendations from the Society for Cardiovascular Angiography and Interventions, the American College of Cardiology and the Heart Rhythm Society were simultaneously published online in the Journal of the American College of Cardiology, HeartRhythm and Catheterization and Cardiovascular Interventions.
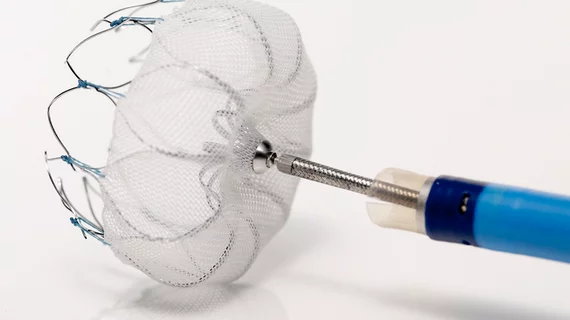
In March, the FDA approved the Watchman device (Boston Scientific) percutaneous closure of the left atrial appendage and to reduce the risk of stroke in patients with non-valvular atrial fibrillation. The device is intended as an alternative to warfarin or novel anticoagulants.
“As this technology becomes clinically available to a broader population of patients, it is essential that physician stakeholders establish criteria for the performance of these procedures that will be used in granting initial and ongoing privileges,” the document’s authors wrote.
For approval and inclusion in the document, at least 70 percent of the writing committee had to approve each recommendation.
The authors noted that physicians should have significant knowledge of atrial fibrillation, the left atrium and left atrial appendage and experience with procedures requiring access to the left side of the heart before performing left atrial appendage occlusion procedures.
Further, they mentioned that institutions that are interested in performing left atrial appendage occlusion procedures should perform at least 50 structural heart disease or left-sided catheter ablations in the past year, of which at least 25 involve transseptal puncture through an intact septum.
Other recommendations include performing the procedures in a cardiac catheterization laboratory or electrophysiology suite, having imaging available for operators, participating in a national registry and reviewing results.
“[This document] will set the standard for safe and effective implementation of this technology to fulfill an important unmet need in treating patients with AFib who are at risk for stroke,” Clifford J. Kavinsky, MD, PhD, FACC, lead author of the document, said in a news release. “[It] will ensure that institutions and operators developing LAA occlusion programs will have the necessary experience, training and infrastructure to carry out this procedure in a way that optimizes patient outcomes.”