FDA panel votes not to endorse Medtronic renal denervation system
Members of a U.S. Food and Drug Administration (FDA) advisory panel have voted not to endorse the premarket approval application (PMA) Medtronic submitted for its Symplicity Spyral Renal Denervation (RDN) System. The announcement came one day after the same panel voted in favor of a PMA submission for Recor Medical’s own RDN system.
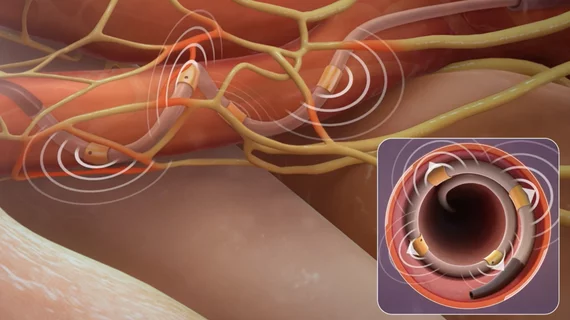
The Medtronic system was designed to reduce blood pressure in patients with uncontrolled hypertension who are unresponsive to traditional medications. It works by using radiofrequency energy to ablate the targeted nerves in a patient’s renal arteries, permanently opening the arteries to improve blood flow.
The FDA’s Circulatory System Devices Panel spent a full day reviewing evidence from the SPYRAL HTN-OFF MED and SPYRAL HTN-ON MED clinical trials and hearing from a number of experts. While the SPYRAL HTN-OFF MED trial did establish that treatment with this device could lead to significant improvements in blood pressure, the SPYRAL HTN-ON MED trial failed to show such an impact.
The advisory panel voted by a count of 13 to 0 that the device was safe to use for patients with uncontrolled hypertension, and it voted by a count of 7 to 6 that the device was effective for treating the intended patient population.
When determining whether the RDN system’s benefits outweighed any potential risks, however, the panel voted by a count of 6 to 6—with the final voter abstaining. Because that vote resulted in a tie, chair Richard Lange, MD, president of Texas Tech University Health Sciences Center El Paso, added his own vote to the conversation. He said his answer to the risks/benefits question was a no, creating a final vote of 6 to 7, with that singer member still abstaining.
“We appreciate the robust conversation that occurred prior to the vote,” Jason Weidman, senior vice president and president of the coronary and renal denervation business at Medtronic, said in a prepared statement after the vote was announced. “We will continue to collaborate with the FDA on bringing a new option to the millions of people living with high blood pressure.”
This does not represent a final decision from the FDA. The agency often votes in line with its advisory panels, but this is not always the case.
FDA advisory panel members discuss their perspective on the Symplicity Spyral Renal Denervation System
One panel member who voted in favor of the Medtronic device for all three questions was Randal Starling, MD, MPH, a cardiologist with Cleveland Clinic. Starling said he felt comfortable with the available data and highlighted the “unmet need” that still exists when it comes to treating uncontrolled hypertension.
“Hypertension is not effectively treated with current tools, and more tools are needed to treat blood pressure,” he said.
Keith Allen, MD, a cardiothoracic surgeon with St. Luke’s Hospital in Kansas City, said he voted “no” to the questions related to efficacy and risks, just as he did for the Reco Medical RDN device the day before. He highlighted the negative results from the SPYRAL HTN-ON MED trial and described the procedure’s efficacy as “mild at best.”
“Sometimes we like to think that the threshold for efficacy should be lowered when the device is very safe, but we still have to think about the overall healthcare burden,” he said.
Another panel member who voted “no” to the second and third question was Julia B. Lewis, MD, a professor of medicine with Vanderbilt Health. Lewis shared her own thoughts about the risks vs. benefits conversation.
“I think we have to be cautious not to confound the desperateness of the unmet need with a willingness to throw anything at that unmet need,” she said. “It’s not going to help people’s blood pressure and their cardiovascular outcomes to have a procedure that is potentially either ineffective or minimally effective.”
The panel’s full discussion about the Medtronic RDN system can be viewed here. Additional details about the panel’s meeting are available here.