FDA panel to review renal denervation system PMA submissions
The U.S. Food and Drug Administration (FDA) has announced that its Circulatory System Devices Panel of the Medical Devices Advisory Committee will review the premarket approval applications (PMAs) for two catheter-based renal denervation systems to treat patients with uncontrolled hypertension. The review is scheduled to take place Aug. 22-23, 2023.
The panel will discuss, make recommendations and vote on the submissions for both the ReCor Paradise Ultrasound Renal Denervation System and the Medtronic Symplicity Spyral Renal Denervation System. Presentations for both devices were among the top late-breaking trials presented at the American Heart Association (AHA) and Transcatheter Cardiovascular Therapeutics (TCT) 2022 meetings. Related presentations for these two devices also headlined previous cardiology meetings in recent years. The FDA panel meeting will be closely watched to see if these devices might be cleared for commercialization by the agency later this year.
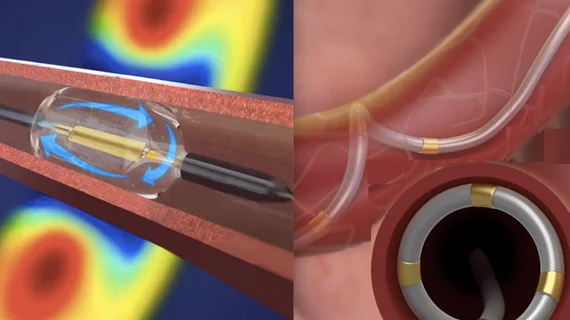
Both devices are designed to treat patients who are inadequately responsive to, or intolerant to, antihypertensive medications. The devices use catheter ablation to kill the sympathetic nerves in the renal arteries. This eliminates vasoconstriction and permanently props open the renal arteries for maximum blood flow to the kidneys. This helps increase filtration of the blood to remove excess water that causes hypertension. The ReCor system uses ultrasound waves and the Medtronic Spyral uses radiofrequency energy to ablate the nerves.
Experts involved with the renal denervation trials say it helps select patients
"So the latest about denervation is that there have been several sham controlled trials that have come out and demonstrated, in comparison to the sham, renal denervation can lower blood pressure," explained Ajay J. Kirtane, MD, director of the cardiac catheterization laboratories and professor of medicine at NewYork-Presbyterian/Columbia University Irving Medical Center, and an investigator in the pivotal RADIANCE-II trial for the ReCor system. "Hypertension is one of our foremost population health issues today, and we need better ways to control blood pressure beyond lifestyle modification and medication. So renal denervation is an adjunct to pharmacology and lifestyle changes."
However, he warned it is not a cure for hypertension, but rather an additional therapy for use in tandem with optimal drug therapy and lifestyle changes. Kirtane said it just adds another tool to the tool box of ways to help patients get their hypertension under control.
"People have to go into this knowing what the blood pressure drop will be, so they don't go into it thinking they are going to be taking patients off of medicines. They will be sorely disappointed because that's not what the study results show," Kirtane explained.
The trials examined very specific patients with uncontrolled hypertension despite optimal medical therapy, but investigators in these trials said the therapy may help other patients beyond these groups.
"I would say adjacent populations, those not necessarily meeting the strict definition of resistant hypertension, should also be considered," explained Deepak Bhatt, MD, MPH, executive director of the interventional cardiovascular program at Brigham and Women’s Hospital, professor of medicine at Harvard Medical School and co-principal investigator for the SYMPLICITY HTN-3 trial for Medtronic's first-generation Simplicity device. He also presented the late-breaking long term data from that trial last fall. "This should include folks with poorly controlled or uncontrolled hypertension, regardless of how they got there. Because even if they are not taking the meds they should, or are eating more salt than they should, the reality is if they come into the emergency room with their blood pressure at 200 over 110, yeah, you can tell them again to take all their medications, cut back on the salt and lose weight. But that patient has a big risk for stroke and other cardiovascular complications, and they are maxed out on drugs and lifestyle modification, doing the best they can. That is real life. Maybe that is the sort of patient this technology can really help."
FDA panel renal denervation meeting details
The Circulatory System Devices Panel of the Medical Devices Advisory Committee is made up of cardiologists, and its purpose is to provide medical opinions and recommendations to the FDA to inform regulatory decisions. The decisions made by the panel are only recommendations, but the FDA usually follows these panel recommendations.
The meeting will be open to the public. Click here for more information from the FDA.