Pfizer recalls 2 drugs, including 1 for hypertension, over safety concerns
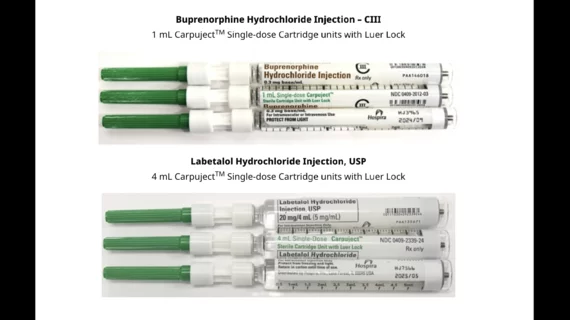
Hospira, an Illinois-based pharmaceutical and medical device company owned by Pfizer, has issued a voluntary recall of two different drugs due to ongoing safety issues. The recall includes specific lots of labetalol hydrochloride injections and buprenorphine hydrochloride injections.
Labetalol hydrochloride injections are used to control blood pressure in patients with severe hypertension.
Buprenorphine hydrochloride injections, meanwhile, are used to help manage pain in patients who require an opioid analgesic and have not responded to alternate treatments.
Pfizer put the recall in place after a customer complained that an incomplete crimp seal had resulted in a leak. These leaks create “potential for an increased risk of lack of therapeutic effect and systemic infection that may result in the need for additional medical treatment,” the company said in a prepared statement.
No adverse outcomes have been reported at this time.
“Pfizer places the utmost emphasis on patient safety and product quality at every step in the manufacturing and supply chain process,” according to the statement. “Pfizer has notified direct consignees by letter to arrange for the return of any recalled product.”
According to Pfizer, any injections identified by customers should no longer be used.
“Wholesalers and hospitals with an existing inventory of any of the lots which are being recalled should discontinue use, stop distribution and quarantine the product immediately,” the company said. “If you have further distributed the recalled product, please notify your accounts and/or any additional locations which may have received the recalled product. Hospitals should inform healthcare professionals in your organization of this recall.”
Click here for a full list of the lots included in this announcement.