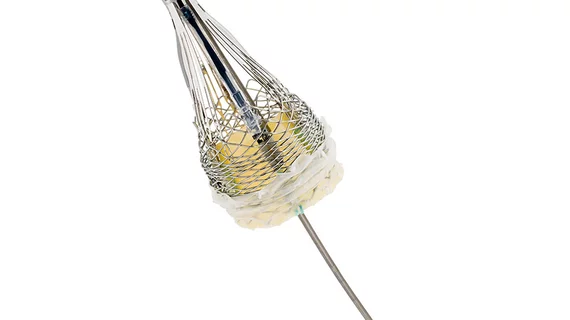
Boston Scientific’s TAVI system exceeds expectations in clinical trial
Boston Scientific’s transcatheter aortic valve implantation (TAVI) technology, the LOTUS Valve System, shows superiority to a similar platform made by a competitor in a new clinical trial.
The results were presented May 16 at the annual EuroPCR Scientific Program in Paris. Boston Scientific, based in Marlborough, Massachusetts, said in a press release that the LOTUS system was shown to be more effective in preventing stroke and paravalvular aortic leakage at one year when compared to a competitor's system.
"The excellent results seen in this large randomized trial, particularly the superior performance in efficacy and the continued demonstration of low PVL rates, further establish the advantages of the LOTUS Valve system," said Ted E. Feldman, MD, a leading investigator on the trial and the director of the Cardiac Catheterization Laboratory at NorthShore University HealthSystem in Evanston, Illinois, in a statement. "With the LOTUS Valve system, I have confidence that I can position the valve accurately in every case and achieve good outcomes for my patients."
The trial, named REPRISE III, is the first pivotal study that has compared two different TAVI platforms. It was a multi-center, randomized controlled trial and included more than 900 patients from the U.S., Europe, Canada and Australia. Each patient had severe aortic stenosis and was considered at high risk for needing a surgical valve replacement.
"We are very excited by the performance of the LOTUS Valve system in this trial as it represents a crucial piece of clinical evidence for the LOTUS platform," said Ian Meredith, MD, executive vice president and global chief medical officer of Boston Scientific, in a statement. "We believe that these data, along with upcoming findings to be shared from the RESPOND and RESPOND Extension studies, can further illustrate the unique clinical benefits that this system offers physicians for the treatment of patients."