Cardiologists share historic research that led to FDA’s long-awaited approval of coronary DCB
The U.S. Food and Drug Administration (FDA) made a decision on March 1 that interventional cardiologists in the United States had been hoping to hear for many years: a drug-coated balloon had finally been approved for the treatment of in-stent restenosis (ISR) in patients with coronary artery disease (CAD).
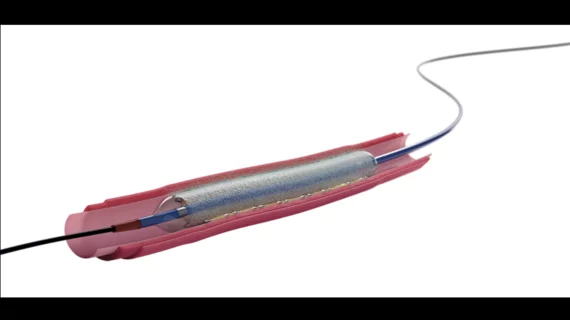
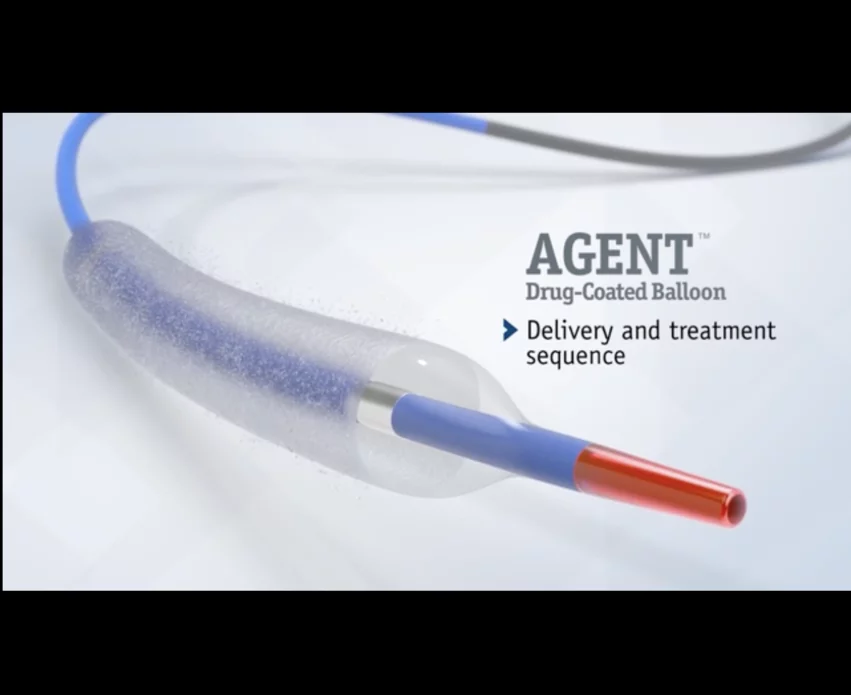
The device in question was Boston Scientific’s Agent Drug-Coated Balloon (DCB), which is used during percutaneous coronary intervention (PCI) procedures to deliver a therapeutic dose of paclitaxel to the patient’s scar tissue and prevent ISR from recurring.
The FDA said its decision was based largely on data from the AGENT IDE trial, which compared treatment with the device to traditional balloon angioplasty. Complete results from that study, however, were not shared with the public until March 9, when they were presented at CRT 2024 and then simultaneously published in JAMA.[1]
Exploring data from the AGENT IDE randomized clinical trial
AGENT IDE included data from 600 patients from 40 U.S. centers who underwent PCI due to coronary ISR. Those high recruitment numbers made it the largest randomized clinical trial ever to focus on the safety and effectiveness of a coronary DCB designed to treat ISR.
The mean age was 68 years, and 73.8% were men. These patients presented with high rates of coronary risk factors and long histories of cardiovascular disease. While 50.7% of patients presented with diabetes, for example, 78.9% had multi-vessel CAD and 30.1% had previously undergone coronary artery bypass graft surgery.
A total of 406 patients were treated with the paclitaxel-coated Agent DCB, and 194 patients were treated with an uncoated balloon. All patients were prescribed dual antiplatelet therapy following treatment.
Overall, clinical procedural success rates (92.1% for DCB patients vs. 88.7% for uncoated balloon patients) and technical success rates (93.4% for DCB patients vs. 89.7% for uncoated balloon patients) were similar. Bailout stents, meanwhile, were required for three patients treated with a paclitaxel-coated balloon and one patient treated with an uncoated balloon.
Target lesion failure after 12 months was seen in 17.9% of DCB patients and 28.6% of uncoated balloon patients. This difference was primarily attributed to lower rates of ischemia-driven revascularization and target vessel myocardial infarction among patients in the DCB group.
“Drug-coated balloons have emerged internationally as an alternative treatment option, but despite promising international data, they have not been previously evaluated or approved for use in the United States,” lead investigator Robert W. Yeh, MD, MSc, MBA, director of the Richard A. and Susan F. Smith Center for Outcomes Research in Cardiology and section chief of interventional cardiology at Beth Israel Deaconess Medical Center, said in a statement. “Even with advances in stent technology, patients with coronary in-stent restenosis continue to comprise approximately 10% of individuals undergoing angioplasty interventions each year. In particular, patients with multiple prior stents have very poor long-term outcomes. There's growing sentiment that drug-coated balloons could address an unmet clinical need among patients with coronary artery disease in the United States.”
Click here to read the full analysis in JAMA, a journal from the American Medical Association.
Interventional cardiologists have wanted this device for years
When the initial positive results from AGENT IDE were presented at TCT 2023, cardiologists were thrilled that a coronary DCB could finally be on its way toward FDA approval.
“Our European colleagues have had these devices for a decade,” Ajay J. Kirtane, MD, an interventional cardiologist with the Columbia University Department of Medicine, said during a TCT press conference in October 2023. “In the United States, we basically tell patients routinely that we can use a peripheral balloon that is too big for your coronary and try to put it in your coronary to prevent the restenosis from happening, or you can buy a ticket to London and go over there to get this treated. It’s embarrassing.”
American College of Cardiology President B. Hadley Wilson, MD, meanwhile, described the new analysis as a “game changer.”
“For 25 years, we’ve been trying to peel back this restenosis problem,” he said during the same press conference. “Now we can see light at the end of the tunnel.”
What’s next now that the Agent Drug-Coated Balloon has received FDA approval?
Boston Scientific said the Agent DCB should be available on the U.S. market in the coming months.
In addition, AGENT IDE’s researchers will be tracking patient data for up to five years, so expect updates on their findings as time goes on.