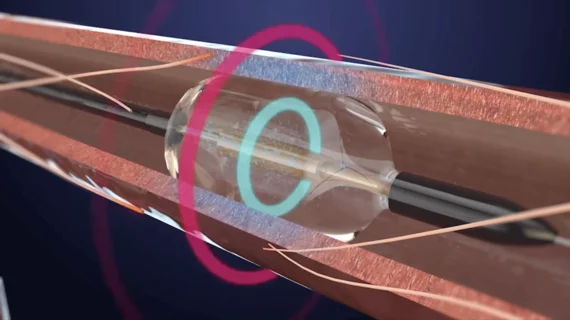
FDA approves first renal denervation system for uncontrolled hypertension
The U.S. Food and Drug Administration (FDA) has approved Recor Medical’s Paradise Ultrasound Renal Denervation (RDN) system for uncontrolled hypertension. This is the first time an RDN system for hypertension has been approved by the FDA.
The system was designed for patients who are unresponsive to traditional medications. It uses ultrasound ablation to kill the nerves in the patient’s renal arteries to eliminate any vasoconstriction. This props the arteries in a full open position, which increases blood flow to the kidneys and removes the excess water that leads to hypertension. Treatment includes two to three seven-second doses of ultrasound energy. Sterile water circulates through the system's balloon catheter to support the patient's renal artery wall and transmit the sonic waves during treartment.
“Recor is leading the way in bringing an innovative solution to clinicians and their patients struggling to control blood pressure,” Laura Barghout, president and CEO of Recor Medical, said in a statement. “This FDA approval is the culmination of years of technical research and rigorous clinical studies. We are grateful to the patients who participated in the studies and to the clinical trial investigator teams whose diligence and dedication made FDA approval possible. We look forward to making this technology available to physicians and their patients nationwide.”
“Approval of the Paradise Ultrasound RDN system marks an important milestone for the company and provides a new adjunctive treatment option for hypertension which remains inadequately controlled despite conventional therapies,” added Noriko Tojo, president of Otsuka Medical Devices, Recor Medical’s parent company.
Renal denervation is not a cure for hypertension
RDN trials over the past decade have had mixed results, despite early high hopes the therapy would offer a clear solution for patients with uncontrolled hypertension. Instead, researchers found it can help reduce blood pressure in patients not controlled by medication, but is an adjunct therapy, not a replacement therapy. Patient selection for RDN is also key.
"I don't think we want to get into a clinical scenario where anybody with high blood pressure is sent for denervation. That is not the right way to approach this," Ajay J. Kirtane, MD, director of the cardiac catheterization laboratories and professor of medicine at NewYork-Presbyterian/Columbia University Irving Medical Center, explained when he spoke to Cardiovascular Business about RDN therapy at AHA 2022. He served as a principal investigator for the RADIANCE-II trial.
"The right way to approach this is to systematically engage with clinicians and teach them how to treat hypertension. I had to learn a lot about hypertension, and I thought I knew it and I said 'how hard can blood pressure be?' It's actually not so easy. So we need to educate in that regard and then treat the patients with lifestyle and medications. And if a patient cannot take the medications, or will not take them, or they are not controlled with them, then denervation can be a good option," Kirtane said.
Click here for the full video interview with Kirtane.
FDA approval for renal denervation system comes months after a key vote
In August, an FDA advisory panel voted in support of Recor Medical’s premarket approval application. The panel’s members reviewed data from three different trials—RADIANCE II, RADIANCE-HTN SOLO and RADIANCE-HTN TRIO—and voted by a count of 12 to 0 that the device was safe to use for patients with uncontrolled hypertension. They panel voted 8 to 3, with one member abstaining, that the system was an effective way to treat patients with uncontrolled hypertension, and voted by a count of 10 to 2 that the benefits of using the system outweigh any risks. The full discussion is available to watch here.
The same FDA panel declined to endorse Medtronic’s own RDN offering, the Symplicity Spyral RDN system, the very next day. That system was designed to work by using radiofrequency ablation to open a patient’s renal arteries. While the panel unanimously agreed the Medtronic system was safe, its members were less sure about its risks outweighing any potential benefits.
These advisory panel votes typically coincide with the agency’s final rulings, but that is not always the case.
Both renal denervation systems already have European CE mark clearance.