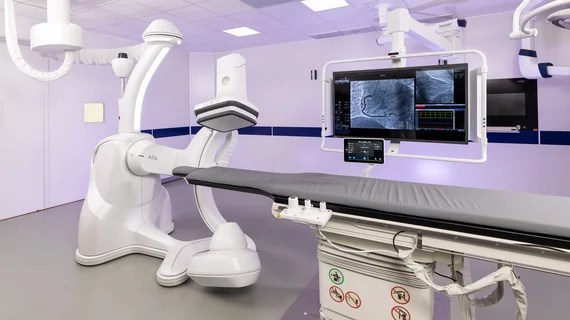
GE Healthcare has received clearance from the U.S. Food and Drug Administration (FDA) for its latest image-guided system (IGS) for interventional cardiology, Allia IGS Pulse. Allia IGS Pulse runs on the company’s Allia Platform and was designed with interventional cardiologists in mind. The angiography system includes a monopolar X-ray tube for acquiring the cardiac images needed to treat of a variety of cardiovascular conditions. It also can capture images of large and bariatric patients who may be associated with certain challenges due to their size.
Allia IGS Pulse also contains an updated version of GE Healthcare’s AutoRight artificial intelligence algorithm, AutoRight Plus, which can adjust up to seven different parameters at once using real-time patient data.
While the FDA was reviewing the Allia IGS Pulse, specialists in France were gaining experience with its features as part of an ongoing pilot program.
“As an operator of the Allia IGS Pulse system, I felt a significant and real improvement in the imaging quality, as well as a significant reduction of this noise in my daily procedures which gave me great confidence and comfort in the operating room,” Nicolas Dumonteil, MD, an interventional cardiologist with Clinique Pasteur in Toulouse, France, said in a prepared statement. “The system is also quite adaptable and versatile to all of my daily situations and procedures – from percutaneous coronary interventions to other complex and structural ones.”
“I saw a significant improvement in the image quality, especially with obese patients and complex angioplasties, where a good visibility of my guidewire, balloons and stents are particularly important,” added Raphaël Philippart, MD, another interventional cardiologist with Clinique Pasteur.
Arnaud Marie, GE Healthcare’s general manager of imaging and guiding systems, explained in the same statement that the company worked to improve its Allia offerings by making improvements in areas where clinicians were still seeing challenges on a regular basis.
“By developing new features to further evolve our core platform, we’re helping to reduce complexity and improve the operating environment so that clinicians can have a personalized workspace that better enables them to keep their focus where it belongs—on their patients,” Marie said.
GE Healthcare will be showcasing the Allia IGS Pulse at the Transcatheter Cardiovascular Therapeutics (TCT) 2023 conference in San Francisco. Click here for more details about TCT 2023.