Initial results promising for world's smallest percutaneous heart pump
The biggest issues limiting percutaneous temporary mechanical circulatory support devices today are the large size of the catheters, the increased risk of vascular complications and the need for higher levels of flow. A new percutaneous left ventricular assist device (pVAD), the Magenta Elevate, may offer a next step with with the smallest catheter size yet to be used in a first-in-human trial.
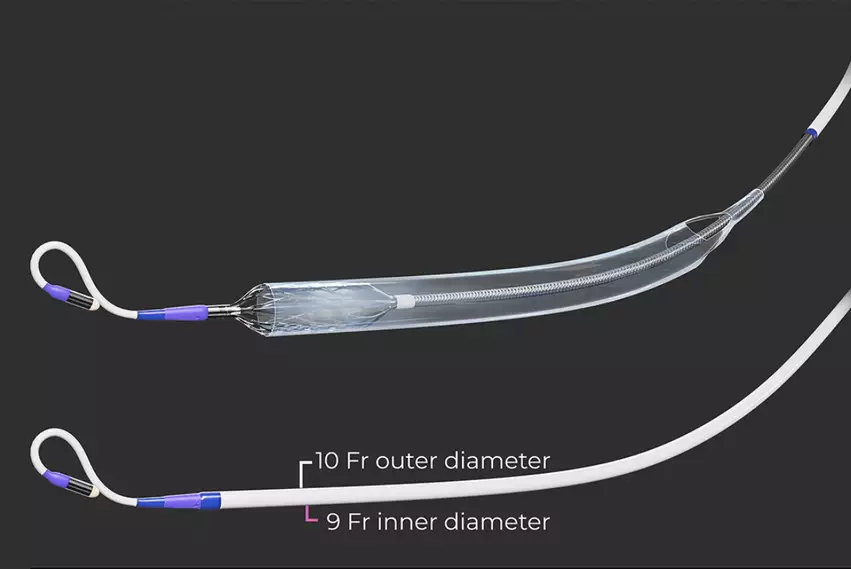
The Elevate uses a 10 French catheter, while the current standard of the Abiomed Impella is 14 French. The new device has the ability to deliver up to 5.5 liters of cardiac output, surpassing the Impella CP's output. There is a 5.5 Impella, but it requires a surgical cut-down. Abiomed is also developing the 9 French ECP Impella system that uses a collapsible cannula, which has completed its pivotal trial enrollment.
Results from the Elevate U.S. early feasibility study were presented at Transcatheter Cardiovascular Therapeutics (TCT) 2023. The study included 15 patients treated at Mount Sinai Hospital, St. Francis Hospital and Heart Center and North Shore University Hospital. The device performance, technical success, safety and usability were all good in the study, with uneventful delivery and extraction of all pumps, no device stoppages or replacement, and no device-related complications.
"Numerous companies are working to bring these devices down to 10 French, with 9 to 10 French being a sweet spot. Balloon pumps are about 7.5 to 8 French, so once you have to 9 to 10 French, you're able to use it in more patients and also expect to have a lower vascular complication of bleeding. And that is exactly what we saw in the first 15 patients," explained Samin Sharma, MD, director of interventional cardiology and director of clinical cardiology for Mount Sinai Hospital in New York, and one of the implanters in the study. Sharma spoke with Cardiovascular Business about his experience and the study results.
The technology was awarded a breakthrough device designation from the U.S. Food and Drug Administration (FDA) for two indications: high-risk percutaneous coronary intervention (HR-PCI) and cardiogenic shock. However, the Elevate does not currently have full regulatory approval anywhere.
Sharma said Magenta is now planning to submit a proposal in 2024 for a randomized trial to compare the device's safety and efficacy with the Impella.
New technology entering the percutaneous VAD space
A few years ago there were high expectations that the St. Jude Percutaneous Heart Pump (PHP) would become the first pVAD to offer a small-diameter catheter and offer higher rates of hemodynamic flow than the Impella. That program was eventually scrapped, however, after issues were encountered with the device.
Sharma, who was involved in PHP studies, said the trials was on again and off again several times due to issues with device leakage, arrhythmias and heart blocks.
In its place, new vendors have come forward with new designs, including Abiomed with its ECP, Magenta, Supira Medical and others. Sharma said Supira, which also has a 10 French device, has already conducted about 20 cases in Brazil. He expects they will start U.S. feasibility cases in early to mid-2024.
This competition from new devices is expected to drive innovation and provide physicians with diverse options for mechanical circulatory support.
Sharma said his cath lab is a high volume centers and does about 15 Impella cases per month. While he thinks Impella has been a great tool and helps patients, there is room for improvement with smaller catheter sizes to prevent vascular complications such as vascular damage and bleeding issues.
He said high flow rates without using a surgical cut down device would also be extremely helpful.
The new devices are creating a lot of excitement in interventional cardiology, and Sharma said he is very interested in seeing the trials unfold and which device will come out ahead as a new standard of care in a few years.
Cardiogenic shock requires higher levels of hemodynamic support
Sharma said cardiogenic shock patients require higher levels of blood flow than the current percutaneous Impella devices can deliver. He said the next generation of percutaneous heart pumps now entering trials will be able to deliver the higher flow rates and use smaller catheters sizes, both of which will likely have a big impact on improving patient outcomes for this high-mortality condition.
"With the Impella ... the output is usually 3 or 3.5 liters per minute, which actually is good enough for patient with a high-risk PCI. But with cardiac shock, you definitely need a high output of 4.5 to 5 liters," Sharma said.