FDA clears new ‘grab-and-go’ interventional device for below-the-knee blood clot removal
Surmodics, a Minnesota-based healthcare technology company, has received U.S. Food and Drug Administration (FDA) clearance for a new interventional device designed to remove thrombi and emboli from below-the-knee peripheral arteries.
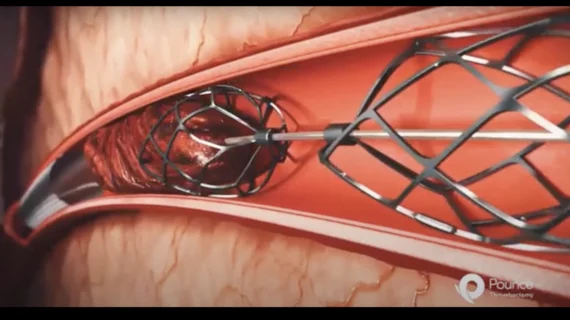
The Pounce LP (Low Profile) Thrombectomy System represents the latest addition to the company’s Pounce platform, a “grab-and-go” solution first launched back in 2021 for the removal of blood clots in peripheral arteries ranging from 3.5 to 6 mm in diameter. The Pounce LP Thrombectomy System works with peripheral arteries ranging from 2 to 4 mm.
“We are excited to secure FDA clearance for the Pounce LP Thrombectomy System, which will extend the range of treatment for our Pounce platform to include removal of organized thrombotic or embolic occlusions in smaller vessels below the knee,” Gary Maharaj, president and CEO of Surmodics, said in a prepared statement. “Catheter-directed thrombolysis in these vessels is limited against organized clot and requires ICU admission, while small-diameter aspiration thrombectomy devices may struggle to remove organized material in the distal lower extremity. By expanding the treatment range of the Pounce platform, we are addressing tibial clots, an important component of treatment in this vulnerable patient population which fills a gap in care.”
Surmodics plans on initiating a limited market evaluation (LME) for the newly cleared device by December 2023. Once the LME is complete, the company plans on full commercial launch of the Pounce LP Thrombectomy System.
The Pounce family of devices include three primary components: a delivery catheter, a basket wire and a funnel catheter. Once delivered, the basket wire deploys two self-expanding baskets that capture the thrombus or embolus and then remove it from the body.
Using real-world data from blood clot patients to build a registry
Back in April 2023, Surmodics announced it had started the enrollment process for its PROWL Pounce Thrombectomy System Retrospective Registry. The registry is designed to gather real-world data from up to 500 patients who have been treated with the company's Pounce platform.
“With hospitals increasingly short on staff and beds, physicians need a simple and effective tool that lets them restore arterial flow right on the table without resorting to time-consuming and costly adjunctive treatments,” Maharaj said at the time in a statement. “We are confident the PROWL registry will demonstrate these exceptional attributes of the Pounce system in real-world clinical practice.”