Blood pressure drug recalled due to black contaminants—FDA highlights safety risks
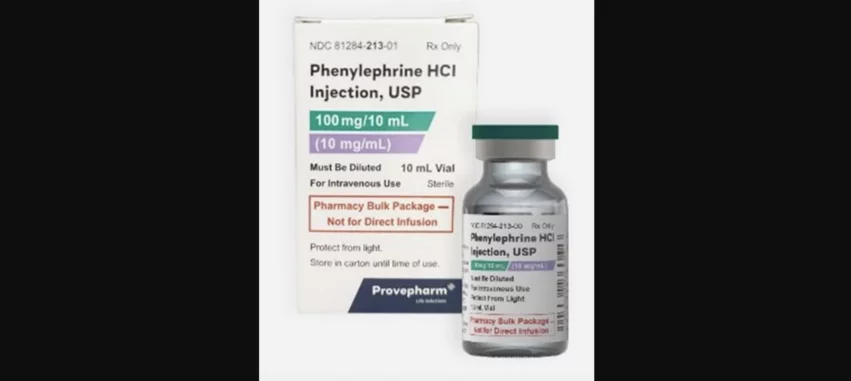
Provepharm, an international pharmaceutical company with U.S. offices in Pennsylvania, has recalled a lot of phenylephrine hydrochloride injections after a customer discovered “visible black particulate matter” in a sealed vial.
“Administration of an injectable product containing particulate matter may cause local irritation or swelling as a response to the foreign material,” according to a recall notice shared by the U.S. Food and Drug Administration (FDA). “If the particulate matter enters the blood vessels, it can travel to various organs and potentially blocking blood vessels in the heart, lungs or brain, leading to serious complications such as stroke or even death.”
Phenylephrine hydrochloride injections are used to treat hypotension, typically in patients under anesthesia being prepped for surgery. The lot included in this recall is sold in vials that contain a single dose, 10 mL, each.
Customers with this product in their possession are instructed to look at the packaging to see if they are impacted by the recall. The lot number is 24020027 and expiration date is December 2025. If any customers do have this product, they should return it immediately to Sedgwick, a company assisting Provepharm with the recall. Click here for Sedgwick’s address and additional contact information from Provepharm and the FDA.
“Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product,” according to the recall notice.