FDA announces another recall of common hypertension medication due to potential cancer risk
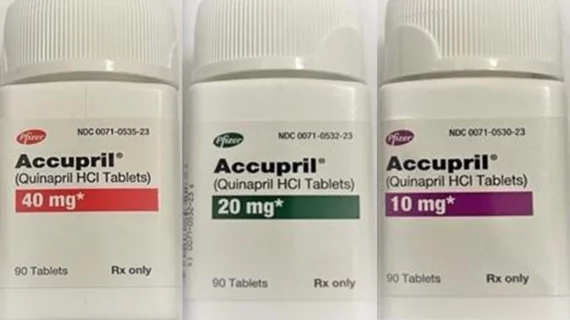
The U.S. Food and Drug Administration (FDA) has announced that Pfizer is recalling five lots of quinapril HCl, a common hypertension medication sold under the name Accupril, due to unacceptable nitrosamine levels. Quinapril HCl is also indicated for the management of certain heart failure patients when combined with conventional therapy.
Nitrosamine, or N-nitroso-quinapril, is regularly found in water and food, but at low levels. Exposure to higher levels, however, has been linked with an elevated risk of cancer, the FDA explained.
“To date, Pfizer is not aware of reports of adverse events that have been assessed to be related to this recall,” according to a prepared statement. “Pfizer believes the benefit/risk profile of the products remains positive based on currently available data. Although long-term ingestion of N-nitroso-quinapril may be associated with a potential increased cancer risk in humans, there is no immediate risk to patients taking this medication. Patients currently taking the products should consult with their doctor or healthcare provider about alternative treatment options for them.”
The voluntary recall products sold in 10 mg, 20 mg and 40 mg packaging. All lots included in the recall were distributed from December 2019 to April 2022. Wholesalers and distributors have been asked to quarantine any recalled products still in their possession.
In March, Pfizer recalled multiple hypertension medications for this same reason. That voluntary recall included quinapril HCI/hydrochlorothiazide tablets, which Pfizer sells under the name Accuretic, and generic quinapril and hydrochlorothiazide tablets distributed by Greenstone.
More from the FDA on nitrosamine impurities
The FDA website includes helpful information about nitrosamine impurities for any patients or healthcare providers hoping to learn more.
“Nitrosamines are common in water and foods, including cured and grilled meats, dairy products and vegetables,” according to the agency. “Everyone is exposed to some level of nitrosamines.”
However, drugs containing nitrosamines “above the acceptable daily intake limits” can increase a person’s risk of cancer over time.
Related Heart Health Content:
FDA announces recall of hypertension medications due to potential cancer risk
Mental illness strongly linked with higher CVD mortality
FDA announces new insulin recall due to potential labeling issue
Renal denervation linked to significant blood pressure reductions after 3 years
COVID-19 linked to a higher risk of diabetes for at least 1 year