Programmable RNA-DNA anticoagulant fibers may provide a better balance between blood clots and bleeding risks
Researchers have developed a new anticoagulant platform that uses programmable RNA-DNA anticoagulant fibers designed to prevent blood clots without leading to unwanted side effects such as in-hospital bleeding events. The therapy offers a novel approach to anticoagulation by telling the body what to do rather than using drug agents.
The group shared its findings in Nano Letters, a peer-reviewed journal published by the American Chemical Society.[1]
“We envision the uses of our new anticoagulant platform would be during coronary artery bypass surgeries, kidney dialysis, and a variety of vascular, surgical and coronary interventions,” Kirill Afonin, PhD, a professor of chemistry at the University of North Carolina at Charlotte (UNC Charlotte), said in a prepared statement. “We are now investigating if there are potential future applications with cancer treatments to prevent metastasis and also in addressing the needs of malaria, which can cause coagulation issues.”
This new research represents the results of a three-year collaboration between UNC Charlotte, the Frederick National Laboratory for Cancer Research, Penn State, Uniformed Services University and the University of São Paulo in Brazil. Afonin described it as a “massive international and interdisciplinary effort to develop a completely new technology that we think may revolutionize the field and be picked up by other areas of health research.”
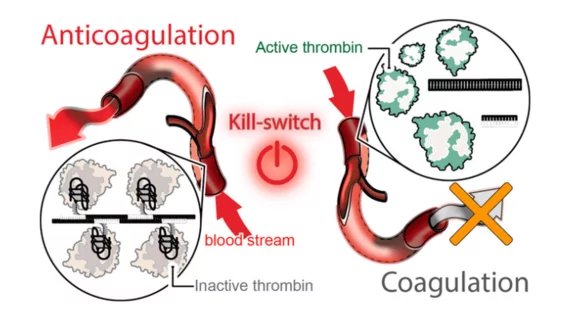
The new treatment platform uses “programmable RNA-DNA anticoagulant fibers” that, when injected into a patient’s bloodstream, are able to communicate with thrombin in their blood. While it prevents blood clotting as needed, its effects can also be reversed by a second “kill-switch” injection. After that second shot, the fibers become inactive and are flushed out of the body.
“We can learn from nature, but we have built something that has never been introduced before,” Afonin added. “So, we develop and build all these platforms de novo—from scratch. And then we can explain through our platforms what we want nature—or our bodies—to do and our bodies understand us.”
![Researchers have developed a new anticoagulant platform designed to prevent blood clots without leading to unwanted side effects such as in-hospital bleeding events. The group shared its findings in Nano Letters, a peer-reviewed journal published by the American Chemical Society.[1]](/sites/default/files/styles/no_crop/public/2022-07/kirill_afonin-file-900px.jpg.webp?itok=SEti2sJW)
