Cardiologists share update after world’s first implant of new optimizer device for tricuspid regurgitation
An 86-year-old patient is showing signs of improvement six months after receiving the world’s first implant of a new-look medical device for treating tricuspid regurgitation (TR), according to new data published in JACC: Cardiovascular Interventions.[1]
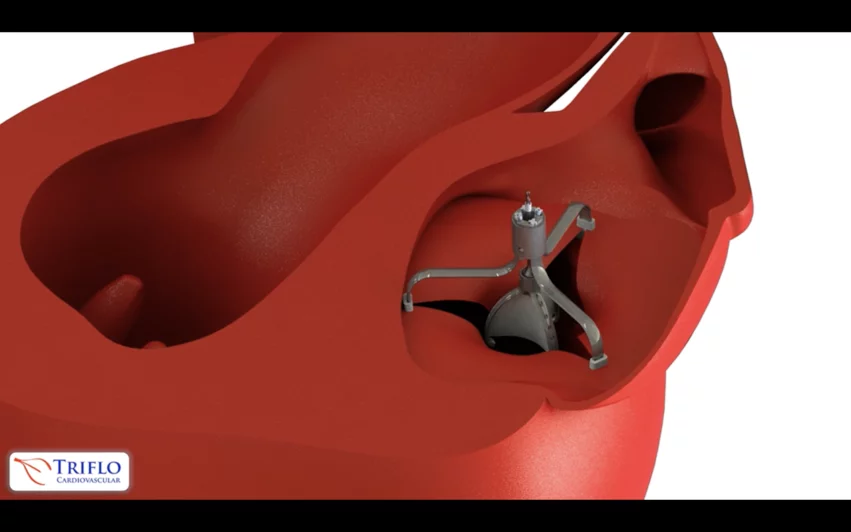
The device in question, the Tricuspid Flow Optimizer, was developed by Triflo Cardiovascular, a U.S.-based biomedical company founded in 2017 by a team of structural heart specialists.
The patient presented with “torrential, symptomatic TR” in addition to a history of atrial fibrillation (AFib) and multiple cardiac surgeries.
“TR was secondary to a posterior leaflet flail, annular dilation and modest tethering, thus the patient was considered suboptimal for available transcatheter therapies,” wrote first author Gian Paolo Ussia, MD, director of the cardiac cath laboratory at Campus Bio-Medico University in Rome, and colleagues. “Following heart team discussion, the patient was accepted for the compassionate use of the Tricuspid Flow Optimizer.”
After using CT and transesophageal echocardiography (TEE) scans to confirm the procedure was feasible, the care team implanted the device. It includes three anchors that are positioned at the tricuspid valve’s commissures. A 37 French steerable catheter was positioned in the patient’s right atrium for the implant, and the device’s positioning was “optimized” before being released. A second TEE scan confirmed the device had been successfully implanted. The patient was discharged after four days of recovery, and a permanent pacemaker was required after three weeks due to slow-rate AFib.
Six months later, the authors reported, reserve remodeling of the right ventricle and a clear improvement in TR were evident.
“The minimal interaction with the right cardiac chamber resulted in an easy implantation of the pacemaker; the polymer leaflets and the minimal footprint demonstrated an optimal adaptation to the native anatomy and stability through six months’ follow-up.”
The Tricuspid Flow Optimizer has not been approved by the U.S. Food and Drug Administration. This was a first-in-human procedure performed in Italy.
Click here for the full analysis.
![An 86-year-old patient is showing signs of improvement six months after receiving the world’s first implant of a new-look medical device for treating tricuspid regurgitation (TR), according to new data published in JACC: Cardiovascular Interventions.[1] The device in question, the Tricuspid Flow Optimizer, was developed by Triflo Cardiovascular, a U.S.-based biomedical company founded in 2017 by a team of structural heart specialists.](/sites/default/files/styles/top_stories/public/2024-04/screen_shot_2024-04-26_at_10.55.10_am.png.webp?itok=3SQu4Esi)