CroíValve kicks off early feasibility study for new TR device, names cardiologist Martin Leon to advisory board
CroíValve, a healthcare technology company with offices in Ireland and the United States, has launched an early feasibility study (EFS) to examine the safety and effectiveness of its new transcatheter device for treating severe, symptomatic tricuspid regurgitation (TR). The announcement comes after CroíValve received the U.S. Food and Drug Administration’s investigational device exemption.
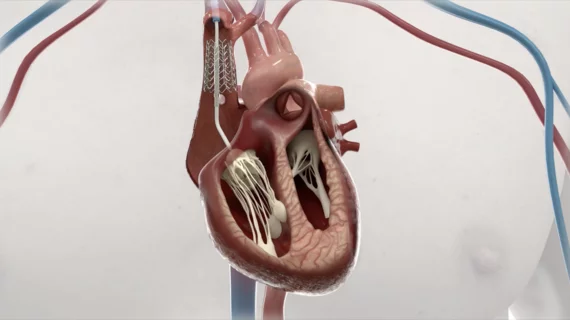
The DUO Tricuspid Coaptation Valve System includes both a prosthetic coaptation valve that works in tandem with the patient’s native tricuspid valve and an anchor system designed to adjust and lock the position of the coaptation valve as needed. According to CroíValve, the device “combines repair and replacement to provide the right solution for the right heart.”
The TANDEM II EFS is expected to enroll up to 15 adult patients with severe or greater TR that remains symptomatic despite medical therapy. All participants will be treated with the DUO system and then be followed for a total of five years. The study’s primary outcome will be freedom from device- or procedure-related major adverse events, which covers death, reintervention, disabling stroke, myocardial infarction, severe bleeding and other potential complications after 30 days.
“We are delighted to initiate TANDEM II, with strong interest in participation in the study from leading U.S. centers. This marks a significant step forward in our efforts to continue generating clinical evidence demonstrating the safety and effectiveness of DUO,” Lucy O’Keeffe, CEO of CroíValve, said in a prepared statement. “We are confident it has the potential to revolutionize the standard of care by redefining how TR is treated, and ultimately enhancing the lives of patients in need.”
Cardiologist Martin B. Leon, MD, joins the CroíValve advisory board
CroíValve also announced that Martin B. Leon, MD, a leading voice in the field interventional cardiology, has been named chair of the company’s clinical advisory board. Leon is a veteran cardiologist and director of the Columbia Center for Interventional Care at New York-Presbyterian Hospital/Columbia University Medical Center. He also founded Transcatheter Cardiovascular Therapeutics (TCT) and serves as chairman emeritus of the Cardiovascular Research Foundation.
“I believe the DUO system can simplify the treatment of TR patients, with a predictable procedure that can be performed with standard imaging techniques,” Leon said in the same statement. “Additionally, with minimal anatomical exclusions, it can reach a broad population. It has the potential to emerge as a meaningful advancement in the field of TR treatment.”