FDA approves new expanding stent for young children
Renata Medical, a California-based healthcare technology company, has gained U.S. Food and Drug Administration (FDA) approval for a new stent specifically designed for pediatric patients with congenital heart defects.
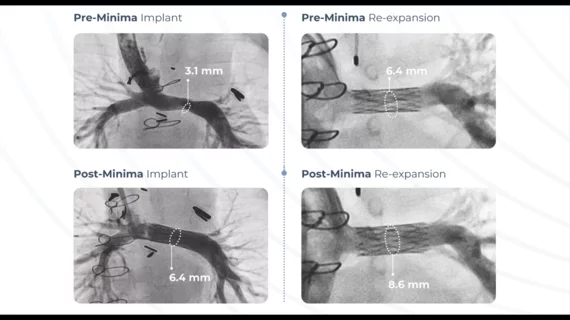
The Minima Growth Stent was built to treat newborns, infants and young children, and then it continues to expand as the patient ages. The FDA’s approval covers both aortic and pulmonary stenosis.
Cardiologists implant the Minima Growth Stent with an interventional procedure that involves access through veins or arteries in the groin or neck. Because those veins are so small in these pediatric patients, Renata Medical built the device to include long, thin struts that can be crimped down to smaller than 2 mm. Most young patients only need to be hospitalized overnight after the procedure before discharge.
“Renata Medical is honored to partner with those helping children born with congenital heart disease,” Dustin Armer, co-founder and CEO of Renata Medical, said in a statement. “The approval of the Minima Stent is a huge milestone for our company, achieving the goal of providing the first stent designed and approved for small, growing children that are unfortunately some of the most vulnerable and overlooked patients.”
“The approval of the Minima Stent is going to have a profound positive effect on our most vulnerable cardiac patients around the world, namely, infants and small children,” added Evan Zahn, MD, director of the congenital heart program at Cedars Sinai Medical Center and chief medical officer of Renata Medical. “Having a minimally invasive stent designed specifically to treat these babies and re-expand to keep pace as a child grows is a monumental advancement for our field.”
The FDA’s decision was based largely on data from the GROWTH pivotal trial, which enrolled 42 patients with a median age of nine months old. Overall, researchers found that 97.6% of patients experienced stenosis relief and no additional surgeries were required six months after implant. In addition, no significant device-related adverse events were reported after six months.
This device is now available in the United States.