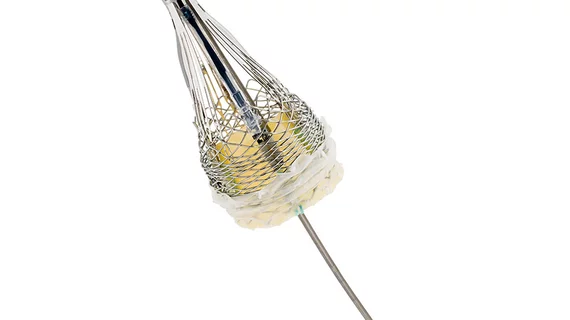
FDA clears LOTUS Edge valve system for TAVR
Boston Scientific’s LOTUS Edge Aortic Valve System was cleared by the FDA April 23 for use in high-risk surgical candidates with severe aortic stenosis.
The LOTUS system, a transcatheter aortic valve replacement (TAVR) technology, is the only such FDA-approved aortic valve that allows physicians the option of repositioning and completely recapturing the valve once it’s been fully deployed, according to a release. Boston Scientific says the valve’s braided nitinol frame and adaptive seal minimizes both paravalvular regurgitation and leaking by conforming to a patient’s native aortic valve.
The valve system also features the company’s proprietary “Depth Guard” technology—designed to reduce left ventricular outflow tract interaction and permanent pacemaker rates by minimizing the depth of the valve during deployment—a flexible catheter and a bovine pericardium.
“Bringing the much-anticipated LOTUS Edge valve system to market allows us to provide patients who aren’t good candidates for traditional surgery a safe and effective treatment alternative to restore proper function to their severely narrowed aortic valve,” Kevin Ballinger, executive vice president and global president of interventional cardiology at Boston Scientific, said in the release. “This technology is a fundamental component of our expanding portfolio and demonstrates our continuing commitment to category leadership within the fast-growing structural heart treatment landscape.”
Boston Scientific said it started a “controlled launch” of the LOTUS system in Europe last month and expects to do the same in the U.S. in the next few weeks.