FDA clears new guidewire used for valve delivery and ventricular pacing during TAVR, BAV procedures
Teleflex Incorporated, a medical device company based in Wayne, Pennsylvania, announced that its Wattson temporary pacing guidewire has received U.S. Food and Drug Administration (FDA) approval.
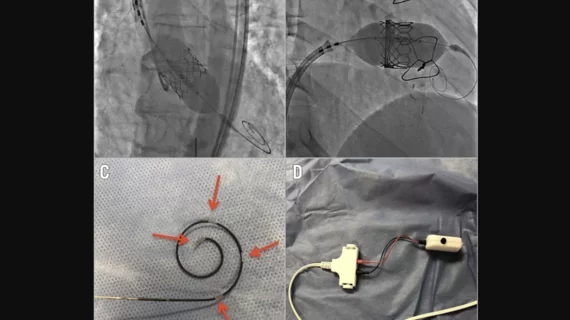
The new-look device, built specifically for transcatheter aortic valve replacement (TAVR) and balloon aortic valvuloplasty (BAV) procedures, helps interventional cardiologists with both valve delivery and ventricular bipolar pacing. Because it requires only one wire to be used, and in only one side of the heart, Teleflex says the device can limit complications, time and healthcare costs compared to other right ventricular pacing devices.
“This technology enables us to provide physicians with a new tool specifically engineered to address unmet clinical needs frequently encountered during TAVR or BAV procedures,” said Jake Newman, president of the Americas for Teleflex, said in a prepared statement announcing the news. “The Wattson temporary pacing guidewire reflects our focus on purposeful innovation and commitment to providing more options to further simplify minimalist TAVR and other structural procedures.”
Back in 2019, researchers shared their early experience with the Wattson device in EuroIntervention, the official journal of EuroPCR and the European Association of Percutaneous Coronary Interventions.[1] The group detailed five patients who underwent interventional procedures with the device; all implants were successful, and no complications were reported.
“In this small series, the Wattson wire offered reliable pacing and rail support for valve delivery in aortic and tricuspid positions,” first author Mark Hensey, MB, BCha, BAO, an interventional cardiologist now with St. James’s Hospital in Ireland, and colleagues wrote. The group added that it could “potentially make TAVR safer and more efficient.”
Teleflex is currently displaying the newly approved device at TVT 2023 in Phoenix. While it is scheduled to enter a “limited market release” in 2023, a full market release is planned for 2024.