SCAI 2021: Harmony TPV could ‘fundamentally alter’ care for patients with congenital heart disease
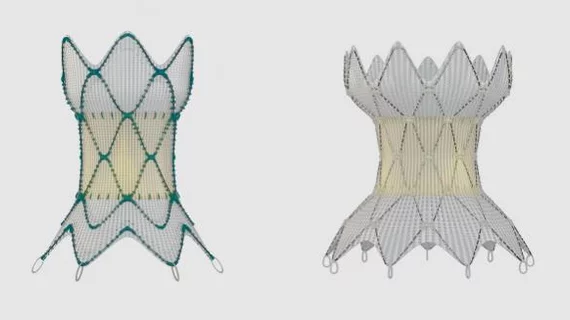
Medtronic’s Harmony transcatheter pulmonary valve (TPV) is a safe and effective treatment option for patients with congenital heart disease and severe pulmonary regurgitation, according to new one-year data presented at the Society for Cardiovascular Angiography & Interventions 2021 Scientific Sessions.
The analysis included 67 patients who received the valve due to an abnormality in their right ventricular outflow tract (RVOT). Harmony TPVs in two different sizes—one with with a 22-mm valve and one with 25-mm valve—were included in the team’s research.
Overall, one-year findings showed that there were no instances of mortality, endocarditis, major stent fractures or surgical intervention among patients who received the Harmony TPV. More than 90% of patients had either no pulmonary regurgitation (PR) at all or just trace amounts of PR when they returned for follow-up appointments.
Two patients did require catheter reinterventions. Several patients also had stable ventricular tachycardia after the procedure, but that resolved in every instance and was not associated with any significant issues.
“This is a brand-new class of cardiac devices designated to help a very specific patient population where no less-invasive, percutaneous treatment options were available until now,” Thomas Jones, MD, the study’s principal investigator and director of cardiac catheterization laboratories for Seattle Children's Hospital, said in a prepared statement. “Unlike any other TPV, this novel technology is designed to expand into the enlarged RVOT in these patients while simultaneously deploying a suitable bioprosthetic pulmonary valve. The Harmony TPV system has the potential to fundamentally alter the lifetime management of CHD patients from here on out.”
The Harmony TPV gained FDA approval back in March. The agency issued a press announcement to highlight the news.
“The Harmony TPV provides a new treatment option for adult and pediatric patients with certain types of congenital heart disease.” Bram Zuckerman, MD, director of the Office of Cardiovascular Devices in the FDA’s Center for Devices and Radiological Health, said at the time. “It offers a less-invasive treatment alternative to open-heart surgery to patients with a leaky native or surgically-repaired RVOT and may help patients improve their quality of life and return to their normal activities more quickly, thus fulfilling an unmet clinical need of many patients with congenital heart disease.”