Medical device company raises $13M for new mitral valve repair technology
CardioMech AS, a Norway-based medical device company focused on structural heart disease, has raised $13 million. This new funding is expected to go toward the continued development of CardioMech’s transcatheter mitral valve repair offering for patients presenting with degenerative mitral regurgitation (MR).
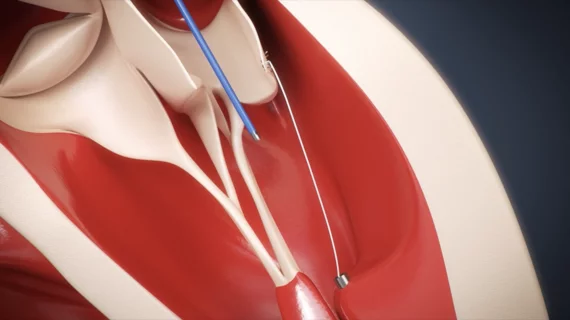
The device in question is an artificial chord intended to reduce or even eliminate MR. Delivered to the patient’s mitral valve via a guide catheter, the repair system was designed to offer clinicians a new noninvasive technique for addressing MR in patients who require an intervention. CardioMech sees the device as an opportunity to treat younger, healthier patients who present with severe MR without performing open heart surgery.
The funding round included contributions from both new and existing investors. To date, CardioMech—which first launched back in 2015—has raised a total of $42 million to develop this new-look technology.
“I am thrilled to continue building on a successful long-term collaboration with our existing investors in the development of this technology, as well as to partner with new individual investors that truly believe in this team and this technology,” Rick Nehm, CardioMech’s president, CEO and chairman of the board, said in a prepared statement. “We are working together to achieve our objective to significantly improve the standard of care for the millions of patients suffering from degenerative mitral regurgitation.”
“CardioMech has the right technology, the right team, the right partners in the right market and we are excited to develop this profound technology,” added Jacob Bergsland, MD, CardioMech’s founder.
The CardioMech mitral valve repair device is still in development. It has not yet been approved by the U.S. Food and Drug Administration for use in the United States.