TAVR system designed to treat aortic regurgitation used in 1st US patient
The first United States patient has been treated with a transcatheter aortic valve replacement (TAVR) system designed to address one of the holes in the rapidly evolving TAVR field: the lack of devices specifically targeting pure aortic regurgitation.
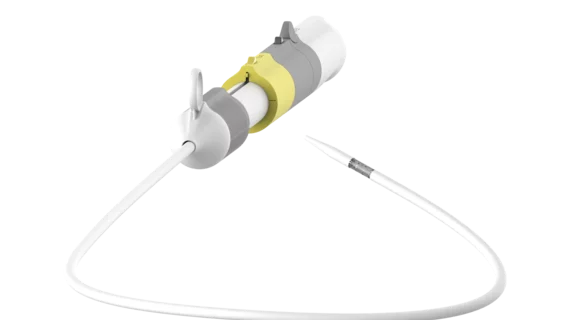
According to device maker JC Medical, the J-Valve TF (transfemoral) system can be used to treat severe aortic stenosis, pure aortic regurgitation or a combination of the diseases. Its unique design features include an anchor mechanism and U-shaped “claspers” which facilitate self-positioning of the bioprosthesis in six different valve sizes.
“The J-Valve TF system has specific attributes that differentiate it from all other currently available TAVR systems, which I believe will enhance the safety and efficacy of TAVR for the indications of aortic valvular insufficiency (regurgitation) and possibly valve-in valve replacement of failing surgically implanted bioprosthetic valves,” Dean Kereiakes, MD, who treated the first U.S. patient with the device at The Christ Hospital in Cincinnati, said in a press release. “J-Valve was clearly the best treatment option for our patient.”
Notably, the JenaValve system is also seeking an indication for TAVR patients with severe aortic regurgitation, with patient enrollment and device implantation for a CE mark study beginning in June 2018. Also, newer-generation TAVR devices were implanted more successfully than early-generation systems in patients with pure native aortic valve regurgitation, according to a study published in December 2017 in the Journal of the American College of Cardiology.
However, those implantations were still considered off-label and the researchers acknowledged there was room for improvement in device development for that challenging population.
According to JC Medical, aortic regurgitation is indicated in more than 20 percent of surgical valve replacements, but zero less-invasive, transcatheter treatment options are approved in the U.S. or Europe for those patients. The J-Valve is available for investigational use only and the company plans to initiate a U.S. clinical trial this year.
“Heart failure due to a defective aortic valve leads to a significant decrease in the quality of life, the loss of life for many, and costs the healthcare system billions of dollars annually,” Ji Zhang, MD, founder and chief technical officer of JC Medical, said in the release. “We look forward to further exploring the promise of J-Valve and its role in treating failing aortic heart valves.”