Boston Scientific TAVR valve comes up short in comparison with Medtronic, Edwards devices
Boston Scientific’s Acurate neo2 Aortic Valve System is associated with worse patient outcomes after one year than other commercially available transcatheter aortic valve replacement (TAVR) valves on the U.S. market, according to new late-breaking data presented at TCT 2024 in Washington, D.C.
Researchers think they have already discovered the primary reason why they were unable to prove noninferiority. Could learning from this mistake help Boston Scientific bounce back from the disappointing results?
Comparing Acurate neo2 with the Evolut and Sapien platforms
The Acurate neo2 gained European CE mark approval years ago, but is not yet been approved by the FDA. For the ACURATE IDE clinical trial, researchers aimed to compare the Acurate neo2 TAVR valve to the Evolut TAVR platform from Medtronic and Sapien TAVR platform from Edwards Lifesciences. The group explored data from 752 patients who received the Acurate neo2 valve, and another 748 patients who received either an Evolut or Sapien valve. Patient characteristics between the neo2 and Evolut/Sapien groups were similar.
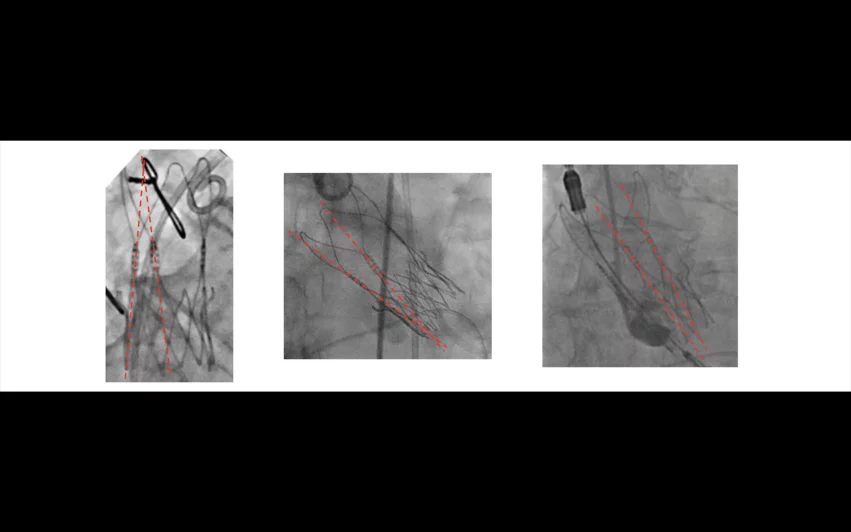
One of the primary reasons the Boston Scientific offering fell short compared to other commercially-available TAVR platforms was the fact that approximately 20% of valves were not fully expanded. Researchers did not notice this trend until they reviewed post-implant angiographic imaging of each patient. Images courtesy of Reardon, et al.
Overall, the study’s primary endpoint—a composite of all-cause mortality, stroke or rehospitalization after one year—was seen in 16.2% of Acurate neo2 patients and 9.5% of Evolut/Sapien patients. A posterior probability for noninferiority of 77.9% was achieved, much lower than the prespecified threshold for noninferiority of 97.5%.
One of the primary reasons the Boston Scientific valve fell short was the fact that approximately 20% of valves were not fully expanded. But, researchers did not notice this trend until a team of engineers blinded to the study’s results reviewed post-TAVR angiographic imaging of each patient.
Valves that are not fully expanded are associated with an increased risk of mortality or stroke—if none of the valves had been under-expanded, or researchers had noticed the trend earlier and warned operators, the trial may have had a very different outcome.
In fact, a sub-analysis of ACURATE IDE confirmed that mortality and stroke rates were comparable between the Acurate neo2 valves and the Evolut/Sapien valves when not including patients who received an under-expanded TAVR valve.
“These data add to the breadth of clinical knowledge of the Acurate valve platform and provide compelling insights on the importance of procedural optimization that will be beneficial for TAVR moving forward,” ACURATE IDE co-principal investigator Michael Reardon, MD, a professor of cardiothoracic surgery at Houston Methodist DeBakey Heart and Vascular Center, said in a statement.
“The data presented today give clinicians a greater understanding of the impact of procedural optimization as the TAVR space continues to rapidly evolve,” added Janar Sathananthan, MD, chief medical officer of interventional cardiology therapies for Boston Scientific. “We believe the findings from the ACURATE IDE post-hoc analysis and implementation of steps to mitigate valve under-expansion may improve outcomes for the Acurate valve and have important implications on other commercially available TAVR valves, and we look forward to studying these improved techniques in future trials of the device.”