Cardiologists are first in world to use new TAVR catheter from Boston Scientific
A new catheter designed to assist interventional cardiologist during transcatheter aortic valve replacement (TAVR) procedures is both safe and effective, according to a new first-in-human study published in JACC: Cardiovascular Interventions.[1]
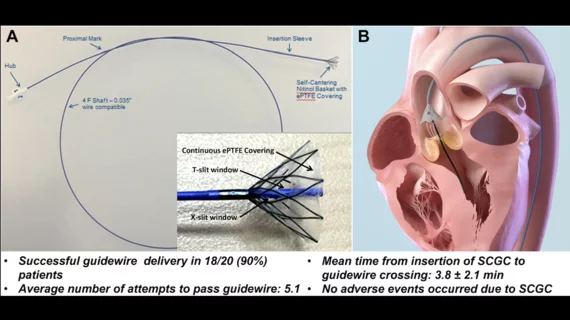
The device in question is the Self-Centering Guide Catheter (SCGC) from Boston Scientific, which was designed to help with the retrograde crossing of a severely stenotic aortic valve (AV) during TAVR. It includes a self-centering basket with a cone-shaped nitinol frame that also has a polytetrafluoroethylene covering and a silicone coating. The basket is captured inside the insertion sleeve and then deployed outside of the insertion sleeve for the crossing procedure. A proximal mark on the catheter shaft indicates when the basket has emerged from the end of the catheter.
“Despite great achievements in the last two decades of TAVR, opportunities for improvement in the procedure continue to exist,” wrote first author Mackram F. Eleid, MD, a cardiologist and echocardiographer with Mayo Clinic in Rochester, Minnesota, and colleagues. “Retrograde crossing of the AV to deliver the guidewire for TAVR is usually performed with a coronary diagnostic catheter, which can be challenging, time-consuming, and can increase the risk of cerebral embolism. Previous efforts to develop catheters for more rapid crossing of the AV during diagnostic catheterization were attempted and not pursued further. The present study is the first to test a purpose-built catheter for retrograde AV crossing during TAVR procedures.”
This first-in-human early feasibility study included 20 patients with severe aortic stenosis. The mean patient age was 78 years old, and 45% of patients were female. The mean Society of Thoracic Surgeons score was 7.2. All patients underwent transfemoral TAVR—18 patients received a balloon-expandable valve, and the remaining two received a self-expanding valve.
A total of 23 SCGC devices were used by four interventional cardiologists to treat the 20 patients. All 100% of the TAVR procedures were a success, and no adverse events were reported.
A guidewire was successfully delivered across the AV into the left ventricle for 90% of patients and 78.3% of the devices used. In addition, the AV was crossed with a guidewire in three or fewer attempts 43.5% of the time, and the self-centering basket was recaptured 100% of the time. The average time from insertion of the SCGC catheter to removal was 6.6 minutes.
“Future device improvements to enhance ease of use, steerability and overall performance are needed, as well as randomized studies comparing this device to existing catheters to study the performance and value in patients undergoing TAVR,” the authors concluded.
Click here for the group’s full analysis in JACC: Cardiovascular Interventions, an American College of Cardiology journal.