Edwards, Abbott share updated TAVR data at CRT 2024
Some of the world’s biggest names in interventional cardiology and structural heart disease gathered in Washington, D.C., for Cardiovascular Research Technologies (CRT) 2024, a four-day healthcare conference focused on advanced cardiovascular technologies and interactive training sessions.
As one might expect, transcatheter aortic valve replacement (TAVR) was one of the most popular topics during the annual event. Multiple vendors used the show as an opportunity to share updated data on their TAVR offerings.
Edwards Lifesciences highlights consistency of Sapien TAVR platform
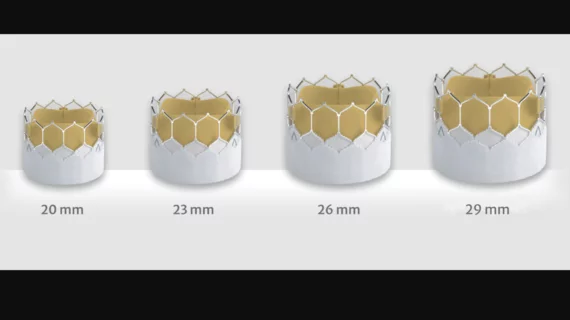
Edwards Lifesciences presented data from two new studies focused on its Sapien TAVR valves. The first study was a comparison of the company’s Sapien 3 Ultra Resilia valve to its Sapien 3 Ultra and Sapien 3 valves; researchers found the performance of each device to be “excellent,” though the Sapien 3 Ultra Resilia valve was associated with a “statistically significant reduction” in paravalvular leak (PVL) outcomes compared to the others.
In addition, the Sapien 3 Ultra Resilia valve was linked to improvements in echocardiography-derived mean gradients, effective orifice area, all-cause mortality and other key outcomes after 30 days.
“These real-world data further add to the robust body of evidence on the performance of Edwards Sapien TAVR and highlight the meaningful advancements of the Sapien 3 Ultra Resilia valve, which provides patients with severe aortic stenosis, the leading option for true lifetime management of their heart valve disease,” Larry Wood, Edwards’ corporate vice president and group president of TAVR and surgical structural heart, said in a statement.
Researchers also shared a new analysis of more than 8,000 patients from more than 800 U.S. facilities who were treated with a 20 mm Edwards Sapien valve. Overall, this smaller valve was associated with three-year mortality and stroke data comparable to the company’s larger Sapien valves.
Abbott’s Portico, Navitor TAVR valves deliver value for high-risk aortic stenosis patients
Abbott, meanwhile, shared new data on both its early-generation Portico TAVR valve and its more recent TAVR offering, the Navitor valve.
First, researchers presented new five-year data on the Portico TAVR valve, the predecessor to Navitor. The analysis included data from nearly 1,500 patients who all faced a high or extreme risk of surgical mortality. Overall, TAVR with Portico was consistently seen as a safe and effective treatment option in high-risk patients. The bioprosthetic valve failure rate after five years was 3%, for example, and severe hemodynamic structural valve deterioration was not seen in a single patient.
A second presentation focused on new one-year data on the newer Navitor TAVR valve, which was designed to include intra-annular leaflets and an active sealing cuff for keeping PVL to a minimum. The study included data from 260 high- or extreme-risk patients treated at one of 26 sites in the United States, Europe or Australia. Overall, after one year, the all-cause mortality rate was 6.6% and the disabling stroke rate was 2.3%. In addition, 99.5% of patients presented with mild or no PVL after that first year.
“For some people diagnosed with severe aortic stenosis, open-heart surgery can be a high-risk procedure due to age, frailty or comorbidities,” Barathi Sethuraman, divisional vice president of clinical affairs for Abbott’s structural heart division, told Cardiovascular Business. “As demonstrated in the pooled analysis of the first-generation Abbott transcatheter heart valve on this patient population through five years, our technology continues to deliver optimal outcomes, including providing excellent and sustained blood flow. Additionally, one-year findings from the IDE study evaluating Abbott’s Navitor, the latest-generation of TAVR system, reaffirms the safety and effectiveness of our technology in reducing or eliminating the risk of blood leakage around the valve implant.”
Additional CRT coverage: Medtronic also shared new TAVR data during the big conference. Read about those presentations here.