FDA approves Medtronic’s next-generation TAVR system with new frame design
Medtronic has received U.S. Food and Drug Administration (FDA) approval for its Evolut FX+ transcatheter aortic valve replacement (TAVR) system for the treatment of symptomatic severe aortic stenosis. The newly approved device represents the latest addition to Medtronic’s Evolut family of heart valves.
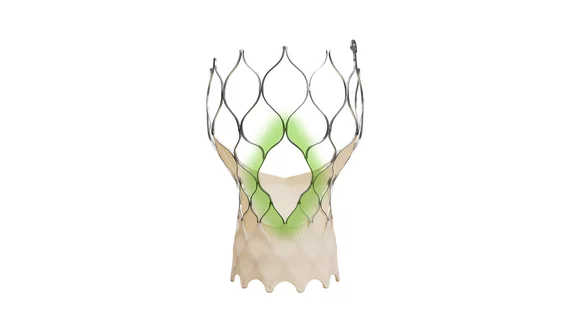
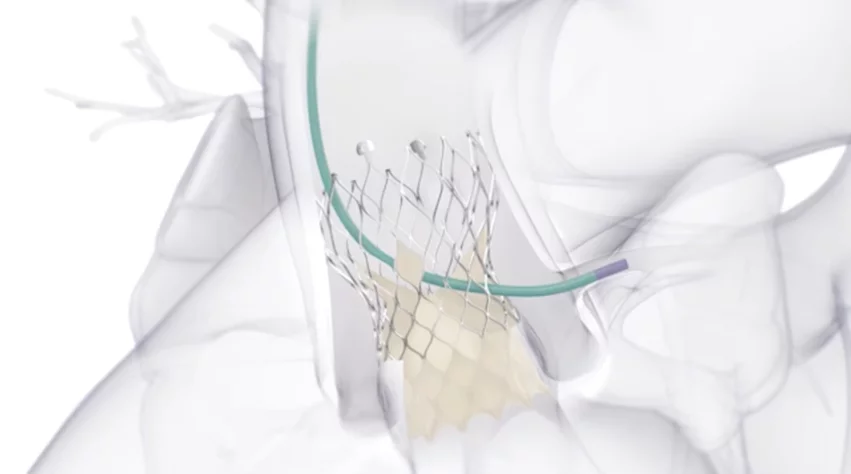
The Evolut FX+ TAVR system includes an updated diamond-shaped frame, which was made four times larger than previous models to offer improved coronary access. It was also designed to provide more space for operators to guide and maneuver the catheter during procedures. According to Medtronic, these modifications were only made when the company could be sure the new device would still be associated with the same valve performance and hemodynamics clinicians associate with the Evolut platform.
“We are committed to consistently developing and advancing minimally invasive solutions for physicians to treat their patients with aortic stenosis,” Jeffrey Popma, MD, vice president and chief medical officer of Medtronic’s coronary and renal denervation business, said in a prepared statement. “This is reinforced by our continued innovation of the Evolut TAVR platform, which has delivered proven valve performance and durability to physicians and patients for years. The Evolut FX+ TAVR system was designed to facilitate coronary access across a diverse range of patient anatomies with no compromise to valve performance.”
The Evolut FX+ TAVR system is approved by the FDA to treat low-, intermediate-, high- and extreme-risk TAVR candidates presenting with symptomatic severe aortic stenosis. While early commercial use is expected in spring 2024, a full launch should follow in the summer months.
More good news for Medtronic’s Evolut family of TAVR valves
This new FDA approval comes just weeks after Medtronic shared positive data on its Evolut devices at Cardiovascular Research Technologies 2024 in Washington, D.C.
One study focused on the economic impact of these valves, finding that TAVR was typically a more cost-effective treatment option than surgery when patients present with severe aortic stenosis. The other study, meanwhile, found that TAVR with an Evolut valve was linked to a lower all-cause mortality/disabling stroke rate (10.7% vs. 14.2%) than surgery and “significantly better hemodynamics.”
“At Medtronic, we continue to emphasize that valve design matters,” Nina Goodheart, a senior vice president at Medtronic and president of the company’s structural heart and aortic division, said at the time. “These data further exemplify Medtronic’s dedication to providing differentiated treatment options like the Evolut TAVR platform to reach the growing population of low-risk symptomatic severe aortic stenosis patients.”