Medtronic receives CE mark approval for Evolut FX TAVR system
Medtronic announced Oct. 12, that its Evolut FX transcatheter aortic valve replacement (TAVR) system gained European CE mark approval.
The device, approved by the U.S. Food and Drug Administration for the treatment of symptomatic severe aortic stenosis (AS) back in August 2021, can now be sold and marketed throughout the EU. Medtronic announced its initial commercial launch in September 2022.
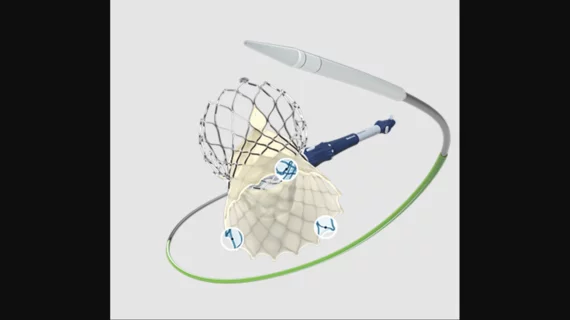
The Evolut FX TAVR system is a supra-annular, self-expanding valve approved to treat both high- and low-risk AS patients. This fourth-generation Evolut device was designed with three gold markers to boost implanter efficiency, an updated catheter tip and a more flexible delivery system than prior Evolut valves.
The gold markers, visible on X-ray imaging, have been perhaps the most publicized of the Evolut FX valve’s new features since the device first launched. In one recent study published in Circulation: Cardiovascular Interventions, for instance, researchers wrote that the radiopaque markers were able to help TAVR operators confirm commissural alignment during TAVR.[1]
“This exciting milestone helps us continually enhance a trusted platform to better respond to clinicians' needs making TAVR procedures easier to visualize and more predictable for heart teams,” Jeffrey Popma, MD, vice president and chief medical officer of Medtronic’s structural heart and aortic business, said in a prepared statement. “Receiving CE Mark for the Evolut FX system highlights our commitment to providing minimally invasive treatment options globally for patients experiencing severe aortic stenosis.”
“With the latest Evolut FX system, we are elevating the precision, control and predictability of transcatheter aortic valve replacement procedures for patients with severe aortic stenosis,” added Danny Dvir, MD, director of interventional cardiology and cath labs at Shaare Zedek Hospital Canter in Jerusalem, Israel. “The system provides physicians with an innovative solution to meet the needs of a patient population desiring to get back to their active lifestyles sooner.”
Now that Medtronic has gained this key approval, the company expects the Evolut FX valve to be commercially available throughout Europe in “the coming weeks.”