New self-expanding TAVR valve shows promise
Researchers have treated a small group of patients with a new-look transcatheter aortic valve replacement (TAVR), sharing their findings in EuroIntervention.[1]
A team in China treated 10 patients—split evenly between men and women—with the SinoCrown TAVR valve developed by Lepu Medical Technology Company. The average patient age was 77.5 years old.
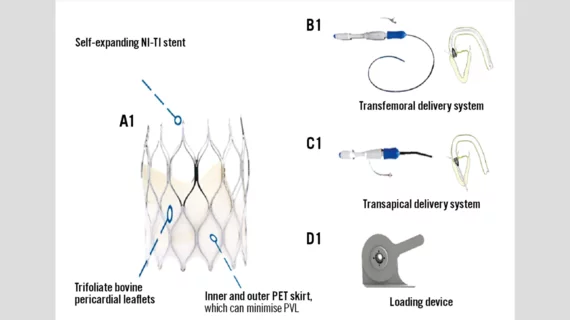
The SinoCrown TAVR valve includes a self-expanding nitinol stent and three leaflets made of bovine pericardial tissue. Three positioning markers are located on the bottom of the valve to help clinicians during the implantation process. Also, the valve’s delivery system was designed to be more flexible and easier to control than other devices on the market.
The SinoCrown valve was associated with a procedural success rate of 100% and a device success rate of 90%. After 30 days, there were no patient deaths, disabling strokes, myocardial infarctions, conversions to surgery or other major procedure-related complications. Paravalvular leak (PVL) was absent in 80% of patients, and the other 20% had just trace levels of PVL.
One patient did require a pacemaker due to a third-degree atrioventricular transmission and complete left bundle branch block.
The team behind the analysis shared their thoughts on using the SinoCrown valve.
“The SinoCrown valve gradually unhooks from the delivery system via the rotary knob, so the valve is completely released and implanted separately,” wrote first author Qingrong Liu, PhD, a cardiologist with Chinese Academy of Medical Science and Peking Union Medical College, and colleagues. “One of its advantages is that it is 100% retrievable; prior to rotating the unlocking knob, the entire valve can be retrieved. According to the pathological conditions of the patient’s peripheral vascular disease, the SinoCrown can be delivered through a transfemoral or a transapical delivery system, both of which are suitable for the majority of aortic stenosis patients.”
Liu et al. also emphasized that it is still too early to make any sweeping comments or recommendations.
“More cases and long-term follow-ups are needed to evaluate the safety and efficacy of SinoCrown,” the group concluded.
This device is not approved to be used in the United States or anywhere else at this time.
Read the full analysis here.