Next-gen TAVR valve, tested on patients for very first time, linked to high success rate
Cardiologists have used a new next-generation balloon-expandable transcatheter heart valve (THV) to perform transcatheter aortic valve replacement (TAVR) on patients for the first time, presenting their findings in JACC: Cardiovascular Interventions.[1]
The valve, seen as a follow-up to the SAPIEN 3 Ultra TAVR valve from Edwards Lifesciences, includes a new frame, new skirt and RESILIA bovine pericardial leaflets. It is currently known as the SAPIEN X4 valve.
“The stent frame is designed to reduce foreshortening and facilitate tailored sizing with expansion across a range of diameters predetermined by selected discrete inflation volumes,” wrote corresponding author John G. Webb, MD, a TAVR specialist with St. Paul’s Hospital and the University of British Columbia, and colleagues. “Thus, the valve has been designed to be increased in size once it is deployed if there is a leak after implantation. Together, the leaflet and frame design optimize leaflet function and durability while maintaining optimal hemodynamic performance.”
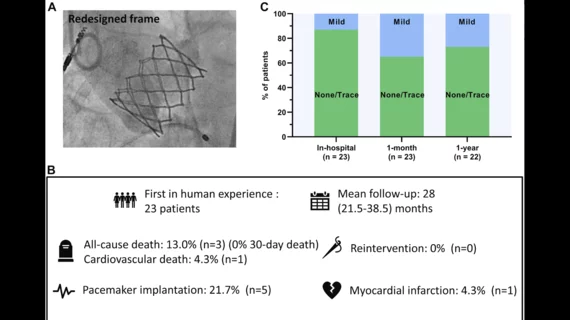
Webb et al.—including lead authors David Meier, MD, and Andrew G. Chatfield, MBChB, both from St. Paul’s Hospital—shared data from a total of 23 patients who underwent transfemoral TAVR using the new-look valve at a single facility from August 2018 to October 2020. The mean patient age was nearly 80 years old, 73.9% of patients were men and the mean Society of Thoracic Surgeons score was 5.5.
The performance was successfully completed for all 23 patients. The median hospital stay was one day.
After one month, no patients had died. One patient did have a stroke, but fully recovered, and another patient “required transfusion due to an access site hematoma.” Permanent pacemaker implantation (PPI) was ultimately required for five patients; the researchers noted that the final implant depth was much deeper in those patients than the patients who did not require PPI.
After a median follow-up period of 28 months, 13% patients had died from causes unrelated to the new valve. One patient was rehospitalized with severe left ventricular dysfunction, but no unplanned valvular interventions were required. All surviving patients were linked to a New York Heart Association functional class of II or lower. In addition, paravalvular leak was not a significant issue for a single patient.
“This first-in-human experience suggests that implantation of this new device is feasible and safe,” the authors wrote. “Favorable clinical outcomes were observed >2 years following TAVR with no death or reintervention related to THV failure.”
The group also emphasized that this was a small analysis. No definitive conclusions can be drawn based on their data, they wrote, and the next-generation valve is still a work in progress.
A much larger study, the ALLIANCE trial, will provide more clarity on the potential of this new-look THV. It is being sponsored by Edwards Lifesciences.