Updated balloon-expandable TAVR valve from Edwards outperforming its predecessors
The Sapien 3 Ultra Resilia transcatheter aortic valve replacement (TAVR) device from Edwards Lifesciences is associated with multiple benefits compared to the company’s previous balloon-expandable valves, according to two different studies published in JACC: Cardiovascular Interventions and EuroIntervention.[1,2]
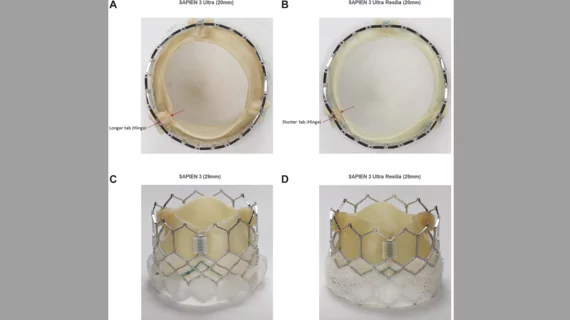
The Sapien family of TAVR valves has been popular among interventional cardiologists for many years now. Edwards designed the Sapien 3 Ultra Resilia with its own anti-calcification tissue technology, which is already found in its surgical heart valves, and included a taller external skirt on the 29-mm option to limit paravalvular leak (PVL).
Multiple research teams have explored the performance of this fifth-generation TAVR valve in recent months. Those studies included:
Tracking real-world outcomes from the STS/ACC registry
Researchers tracked data from the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry, focusing on two propensity-matched groups of 10,314 TAVR patients. While one group underwent treatment with the Sapien 3 Ultra Resilia device, the other group was treated with the Sapien 3 or Sapien 3 Ultra devices previously developed and manufactured by Edwards. The team shared its analysis in JACC: Cardiovascular Interventions.
“The refinements incorporated into the Sapien 3 Ultra Resilia transcatheter heart valve design offer potential clinical advantages, especially as TAVR shifts toward treatment of younger and lower-risk patients,” wrote first author Curtiss T. Stinis, MD, an interventional cardiologist with Scripps Clinic in California, and colleagues. “There is an increasing focus on lifetime management of aortic stenosis with the acknowledgment that many patients currently being treated with TAVR will live long enough to develop clinical valve failure necessitating repeat aortic valve intervention. As such, a transcatheter heart valve design that provides extended durability offers patients the potential for fewer needed lifetime aortic valve interventions.”
After 30 days, the rates of death, stroke and bleeding events were all comparable. At discharge, meanwhile, the Sapien 3 Ultra Resilia valve was associated with significantly lower mean gradients (9.2 mm Hg vs. 5.7 mm Hg) and a larger mean aortic valve area (2.1 cm2 vs. 1.9 cm2). The study’s authors also highlighted the reduced PVL seen with the 29-mm valve, a result of that updated skirt design.
“Continued follow-up is needed to assess long-term outcomes and valve durability,” the authors added.
Read the full study here.
OCEAN-TAVI study explores impact of new technology on patients in Japan
In a separate study, researchers compared data from 618 consecutive TAVR patients treated with the Sapien 3 Ultra Resilia device with a pooled cohort of nearly 9,000 TAVR patients treated with one of the previous Sapien 3 valves. All data came from OCEAN-TAVI, an ongoing look at patients treated in 15 different hospitals throughout Japan. The team shared its findings in EuroIntervention.
Overall, the group found, treatment with the Sapien 3 Ultra Resilia valve was linked to comparable rates of procedural complications, lower rates of PVL and patient prosthesis mismatch (PPM) and “significantly better valve performance.”
“Importantly, the rate of PPM after Sapien 3 Ultra Resilia transcatheter heart valve implantation is significantly lower, even after propensity score matching and even with the use of smaller 20-mm and 23-mm Sapien 3 Ultra Resilia transcatheter heart valves,” wrote first author Masanori Yamamoto, MD, an interventional cardiologist with Toyohashi Heart Center in Japan, and colleagues. “Overall, the latest-generation S3UR has considerably improved the previous limitations of balloon-expandable transcatheter heart valves.”
Read the full study here.