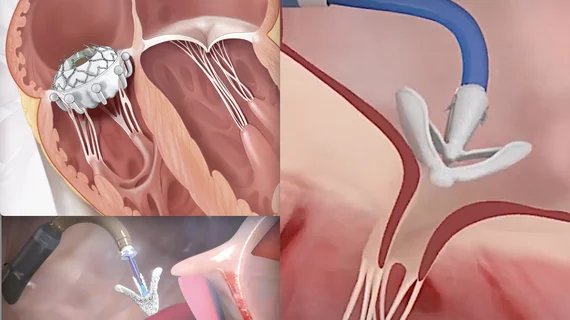
Tricuspid valve therapies moving ahead of mitral therapies seeking market approval
After the stellar success and rapid adoption of transcatheter aortic valve replacement (TAVR), it was widely expected that same rapid development and success story would follow with the mitral valve, which makes up the bulk of heart valve repair and replacement procedures. However, the complexity of the mitral anatomy has slowed this area of development, and it now seems much more likely transcatheter tricuspid valve repair and replacement (TTVR) devices will push ahead to gain U.S. Food and Drug Administration (FDA) clearances in the next year or two.
This has led to a large shift in interest for tricuspid valve therapies. Experts in structural heart say several TTVR devices will soon be poised for final FDA review, which will open new business opportunities for structural hearts centers. This trend was apparent during the 2022 Transcatheter Valve Therapies (TVT) Structural Heart Summit in June and will also be a seen at the annual 2022 Transcatheter Cardiovascular Therapeutics (TCT) meeting in September.
"When we talk about when a mitral transcatheter replacement valve that might gain FDA approval, we are talking about three or four years. But when we come talk about the tricuspid valve, it is completely flipped around," explained Azeem Latib, MD, section head and director of interventional cardiology and the director of structural heart interventions for Montefiore Health System. "The tricuspid has completely revolutionized the way we are thinking about valves because the space has grown incredibly quickly. This s because of a couple of reasons. First, it is important and we should not forget about it and we should stop calling it 'the forgotten valve.' Two, these patients have very few options."
The tricuspid has been nicknamed the "forgotten valve" for years, because it was largely ignored due to poor surgical outcomes, explained Anita W. Asgar, MD, MSc, FSCAI, director, transcatheter valve therapy research at Montréal Heart Institute. But with minimally invasive transcatheter procedures now being tested in trials, the new approach has breathed new life and interest into the tricuspid valve.
"In the tricuspid, we don't have this left ventricular outflow tract (LVOT) obstruction issue like we do with the mitral valve," Asgar explained. "We may have sizing issues and AV block issues that require pacemakers, but we realized there are a lot of these patients out there. The surgical outcomes are less optimal that mitral valve surgery outcomes, so this is really a population that has an unmet need. The surgical data on valve replacement is not great, but what we are seeing with transcatheter valve placement is very encouraging."
Tricuspid regurgitation affects an estimated 1.6 million people in the U.S., increasing as each generation ages. But like TAVR, it is possible there are actually more patients in needs of tricuspid interventions who have never made it to surgeons before because there were not many good options for therapy. Once TAVR became available, much larger numbers of aortic valve patients came out of the woodwork seeking the minimally invasive therapy, boosting overall aortic vale replacement procedures, both surgical and TAVR and most centers.
"Tricuspid regurgitation is a big and growing problem for us," explained the Adam Greenbaum, MD, co-director, Structural Heart and Valve Center, Emory University Hospital Midtown, Atlanta. "The mortality and morbidity of severe symptomatic tricuspid regurgitation is significant, which a 30% mortality rate at one year after surgery. We don't have great therapies for them, aside and aside from diuretics, we don't have much to offer them. And surgery is associated with morbidity and mortality because the patients are so sick, they are frail and they are elderly. This this has opened the door for the potential benefits for transcatheter therapies."
Anatomical issues with the mitral valve have slowed TMVR development
The aortic valve was first targeted for transcatheter interventions because it was the easiest to access and straightforward to treat. However, the mitral valve is the most complex valve because of its anatomy and physiology. Asgar said the mitral valve is more of a "D" shape, so it is harder to create a valve that fits. She added it has a very small landing area to place a valve and has tethering cords attached to the leaflets that can get tangled in any devices placed or navigated there. Any transcatheter device placed in the metal annulus also needs to have a very small profile to prevent over hang into the LVOT, otherwise it will become a blood flow obstruction. Anchoring a valve in this high pressure, dynamic environment also requires different strategies beyond just outward expansion of the valve body used in TAVR. This has led to several valve systems that use much more complex anchoring systems to clip the edges of the valve to the annulus, or sandwich the native valve between two sections of a transcatheter valve.
"One of the things we have learned is that mitral anatomy is much more complex than aortic, so there have been many different strategies and technology developed to implant a mitral valve," Asgar explained.
This complexity also varies from patient to patient, requiring intense pre-procedural screening to plan implants and decide if a patient's anatomy is suitable for a transcatheter valve implant.
"Depending on the device, you can have screening failure rates of between 20% to 65%, based on the size of the valve, the way it anchors and what the impact is on the LVOT," Asgar explained. "Some of these devices are better suited for calcium, although the majority of the devices are being targeted for mitral regurgitation."
Latib noted the first MitraClip device was implanted in a patient in 19 years ago in 2003, and today it remains the only FDA cleared transcatheter device to treat the mitral valve. He said the fact no other devices have come to market it directly related to the complexity of the mitral valve.
While these issues are being worked out in the mitral space, she said the tricuspid valves does not have these same issues. While harder to access and having its own set of challenges, Asgar said the tricuspid valve is technically easier to treat than the mitral valve, which is what led to devices in the TTVR space pushing ahead of mitral devices seeking regulatory approval.
Transcatheter tricuspid valve technologies to watch
The rapid advance in tricuspid technology is evident in Europe, where there are already three transcatheter catheter repair devices approved. These include the Abbott TriClip, Edwards Pascal and the Edwards CardioBand. Latib expects the first fully implantable transcatheter tricuspid valve to be cleared in Europe in the next few months.
"That is incredible if you think about the short time it has taken to get to that compared to mitral," Latib said.
In the U.S., he said there are three FDA pivotal trials on the way that could pave the way toward the first FDA device approvals. These include the TRILUMINATE trial for the TriClip, the PASCAL TR trial for the Pascal clip device, and the TRISCEND II clinical trial for the Edwards Evoque transcatheter tricuspid valve.
"These studies are enrolling incredibly quickly and two of them are close to finishing, so I suspect in the next one to two years we will have the first approved devices in the United States for tricuspid regurgitation," Latib said.
Latib said the tricuspid valve is complex, similar the mitral valve, but it has some advantages making it easier to design devices and get trials completed more quickly.
"There are important differences that have made it easier for designing valves," Latib explained. "The fact that we are dealing with a lower pressure state compared to the mitral, and there is no risk outflow tract obstruction, which makes it easier to put valves in the right side of the heart."
The results with these valves have also been fantastic. "The patients go from torrential tricuspid regurgitation to zero with the valve procedures, so it has been really phenomenal," Latib said.
Realizing the possible rapid advances that can be made in the tricuspid space, the FDA has been very open to working with physicians on to answer many remaining questions of the valve to speed the science and approvals of devices, Latib said. He delivered the keynote in the FDA session at TVT.
Greenbaum presented the one-year data from the CLASP TR trial feasibility study for transcatheter edge-to-edge repair (TEER) using the Edwards Lifesciences Pascal at the 2022 American College of Cardiology (ACC) meeting. He said patients in the trial saw significantly reduced severity in their tricuspid regurgitation and improved their ability to function in daily life. These benefits were maintaining at follow-up. He said they are already prepping for the CLASP II TR study, which will widen enrollment to 825 patients.
Similar good results have come from the Abbott trials for its TriClip, a tricuspid version of its MitralClip device.
Despite the fast pace of transcatheter tricuspid valve repair and replacement development, Latib said there are still factors that are unknowns because this therapy space is so new.
"I realized when writing the keynote for the FDA session at TVT, there is still so much we don't know and many questions we still need to answer, and it has been great how the FDA has been open to working with physicians an scientists to try and answer those questions," Latib said.
He said questions that remain include what is the best measure for success in patients. Is it mortality benefit, reducing hospitalization or just making patients feel better, Latib said. That will be worked out in future trials and registries.