FDA announces new recall of neurovascular catheters built by Johnson & Johnson subsidiary
The U.S. Food and Drug Administration (FDA) has announced that Medos International Sàrl, a Swiss manufacturing company owned by Johnson & Johnson, is recalling more than 1,000 neurovascular catheters due to concerns about the devices breaking.
This is a Class I recall, which means the FDA believes using these devices “may cause serious injuries or death.”
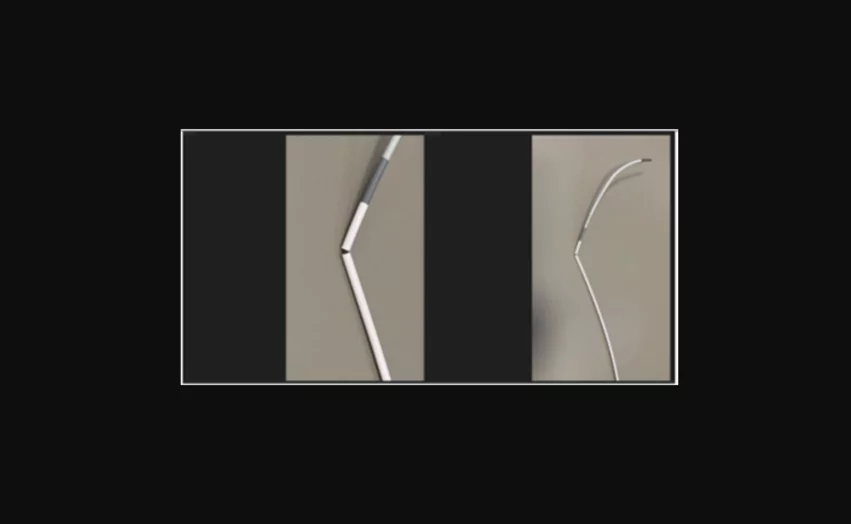
Medos International Sàrl is recalling the Cerebase DA Guide Sheath, a catheter typically used when accessing blood vessels in the brain to deliver interventional devices, after receiving multiple complaints about fractures in the device’s distal catheter shaft.
“The use of the affected product may result in surgical procedural delay, vascular injury or hemorrhage, and in extreme rare occasions it may result in embolism,” according to the FDA advisory.
Three patients have been injured as a result of this issue. No deaths have been reported.
The recall includes a total of 1,343 devices manufactured by Medos International Sàrl. They were distributed to customers from July to December 2023. The devices are sold by Cerenovus, a Johnson & Johnson MedTech company focused on stroke care.
Medos International Sàrl and Johnson and Johnson issued an Urgent Medical Device Recall notice to all impacted customers in February 2024. The companies recommended that all customers go through their interventional device inventories and quarantine any products included in the recall. Arrangements can be made to return them back to the manufacturer.
Additional details about this new Class I recall are available on the FDA website.
Another significant catheter recall
This news comes just one day after the FDA announced Arrow International, a subsidiary of Teleflex, is recalling more than 300,000 catheterization kits due to ongoing safety concerns. That was also viewed as a Class I recall due to the seriousness of the potential risks. Arrow International had received 194 complaints about the issue; it was associated with 10 injuries and one patient death. Read more.