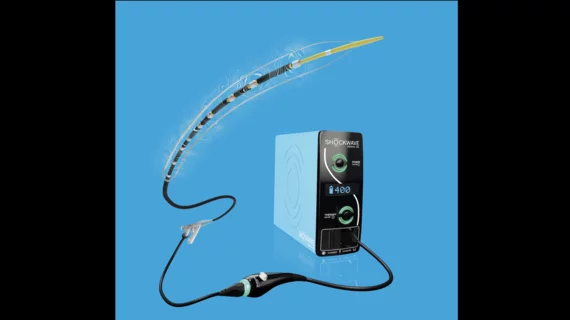
Shockwave Medical launches new FDA-approved IVL catheter for PAD, CLTI
Shockwave Medical, a Johnson & Johnson MedTech company, has officially launched its Shockwave E8 Peripheral IVL Catheter in the United States.
Johnson & Johnson recently acquired the intravascular lithotripsy (IVL) company for approximately $13 billion. While many other Johnson & Johnson acquisitions underwent name changes and now go by Johnson & Johnson MedTech, that is not yet the case for Shockwave.
Shockwave’s E8 catheter was designed specifically to treat patients with calcified femoropopliteal and below-the-knee (BTK) peripheral artery disease (PAD), including those diagnosed with complex chronic limb-threatening ischemia (CLTI). It delivers 400 pulses twice per second, has a working length of 150 cm and includes eight emitters across a single 80-mm balloon.
The U.S. Food and Drug Administration (FDA) previously cleared the E8 catheter, and now Shockwave has made it available throughout the U.S. market.
“Shockwave's newest peripheral catheter offers significant improvements that will help physicians refine their treatment algorithm and better support challenging patients with heavily calcified disease,” Venita Chandra, MD, a vascular surgeon familiar with the device and clinical associate professor with the division of vascular surgery at Stanford Health Care, said in a statement. “The catheter's ability to treat long lesions and its extended reach enable safe and effective treatment of some of our most difficult-to-treat patients, including those with CLTI, a complicated and severe disease state with a high mortality rate.”
“Shockwave E8 represents our commitment to physician-guided innovation while maintaining the simplicity and effectiveness of our IVL technology,” added Isaac Zacharias, president of Shockwave Medical. “We have received very positive physician feedback on Shockwave E8 and look forward to establishing it as our new workhorse peripheral IVL catheter for physicians addressing challenging calcium above and below the knee.”
This latest launch officially expands the Shockwave Medical peripheral portfolio, which already includes the FDA-approved Shockwave L6, Shockwave M5+ and Shockwave S4 IVL catheters.