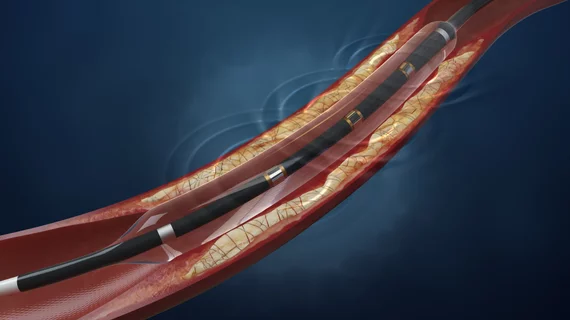
CMS recommends additional payment for using Shockwave’s IVL technology in hospital setting
CMS has ruled that coronary intravascular lithotripsy (IVL) cases performed in a hospital setting be eligible for an additional payment through a New Technology Add-On Payment (NTAP).
The proposal is included in the Fiscal Year 2022 Hospital Inpatient Prospective Payment System (IPPS) proposed rule, which was published April 27 and is currently open for public comment.
Shockwave Medical, the healthcare technology company that started developing IVL back I 2009, issued a statement in support of the recommendation.
“We applaud CMS for proposing an NTAP for Shockwave’s coronary IVL and for their commitment to improving Medicare beneficiary access to breakthrough technologies,” Robert Fletcher, vice president of marketing and market access for Shockwave Medical, said in a prepared statement. “This is another step forward in our early introduction of coronary IVL and in our mission to expand access to new technology that addresses a significant unmet need in the treatment of complex coronary artery disease.”
Shockwave’s IVL technology has gained FDA approval for the treatment of severely calcified coronary artery disease and peripheral arterial disease.
Click here to read more about IVL in the Cardiology Business digital magazine. More information on the IPPS proposed rule is available here.
Related Content on Intravascular Lithotripsy to Break Up Calcified Lesions:
VIDEO: Shockwave Medical demonstrates faster intravascular lithotripsy at ACC22
Shockwave’s IVL technology gains FDA approval for treating advanced coronary artery disease
Multicenter trial to evaluate IVL treatment for peripheral lesions below the knee
Better Together? An Integrated Market for Calcium-modification Strategies
IVL Turns Standard Balloon Technology into a Powerful Calcium-disruption Tool