Edwards Lifesciences to acquire medical device startup behind new TMVR technology
Edwards Lifesciences has agreed to acquire Innovalve Bio Medical, an Israel-based medical device company focused on structural heart disease technologies. Financial terms of the deal have not been announced to the public.
Edwards had an open option to acquire Innovalve after initially investing in the company back in 2017. It chose to exercise that option after Innovalve “demonstrated progress” with “promising” early results.
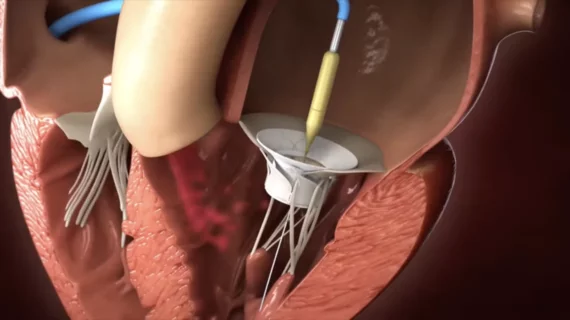
Innovalve’s primary focus is its new Innostay transcatheter mitral valve replacement (TMVR) system, a minimally-invasive device designed to treat patients with severe mitral regurgitation (MR). The Innostay TMVR system rotates in a way that produces “robust anchoring and excellent sealing.” According to Innovalve, it can be deployed quickly, leading to short procedure times, and was built to work with mitral valves of any size.
“Building on our learnings of the complexity of mitral disease, we know there is a need for a differentiated range of therapies for these patients,” Daveen Chopra, corporate vice president of the Edwards transcatheter mitral and tricuspid therapies product group, said in a statement announcing the news. “Edwards’ Sapien M3 remains on track to become the first approved transfemoral TMVR system in Europe by the end of 2025. We believe the Innovalve technologies, paired with Edwards’ deep mitral expertise, will enable a TMVR platform that will expand the treatable population.”
The Innostay TMVR system is not yet approved for use in the United States by the U.S. Food and Drug Administration, but Innovalve is actively recruiting patients for the TWIST early feasibility study. The trial will include MR patients treated with the device in both Israel and the United States. U.S. locations include Cleveland Clinic and NYU Langone in New York City.
“Data collected in this clinical trial will include 30-day safety and performance of the device and delivery system, and long-term clinical follow-up over a five-year period,” according to Innovalve.