Johnson & Johnson to acquire heart failure specialists V-Wave for up to $1.7B
Johnson & Johnson has agreed to acquire V-Wave, an Israeli medical device company focused on developing advanced heart failure technologies, for an upfront payment of $600 million. If specific regulatory and commercial milestones are met, the deal could include payments totaling an additional $1.1 billion.
Once this acquisition is finalized, V-Wave will become part of Johnson & Johnson MedTech. Johnson and Johnson initially invested in V-Wave back in 2016.
“We are excited to welcome V-Wave to Johnson & Johnson MedTech and to take another meaningful step toward transforming the standard of care for cardiovascular disease,” Tim Schmid, executive vice president and worldwide chairman of Johnson & Johnson MedTech, said in a statement. “We recognize the importance of identifying more diverse and effective treatments for heart failure, and our recent track record demonstrates our focus on accelerating our impact on the most urgent and pressing unmet needs.”
“We are confident that Johnson & Johnson MedTech is well-positioned to ensure V-Wave’s breakthrough ideas and technology reach patients in need as quickly and effectively as possible,” added Neal Eigler, MD, V-Wave’s CEO. “I couldn’t be prouder of the V-Wave team, and the commitment it has taken to achieve this milestone.”
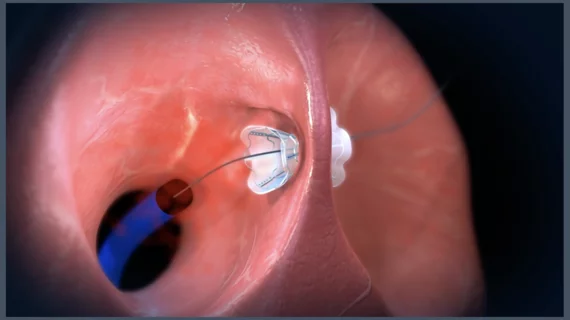
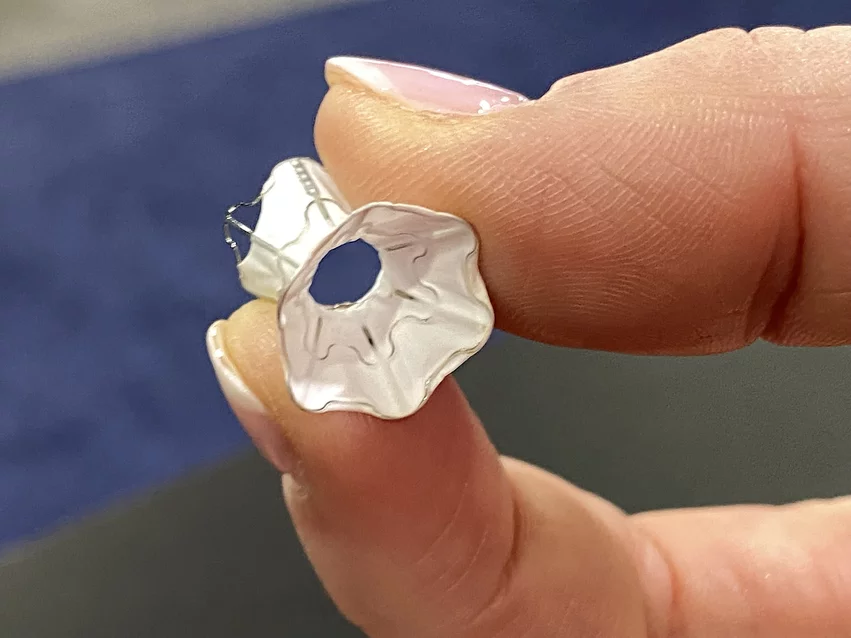
How V-Wave’s implantable Ventura device targets HFrEF
V-Wave has gained considerable attention for its Ventura Interatrial Shunt System, a small implantable device designed to reduce pressure on the left atrium and the lungs in patients with heart failure with reduced ejection fraction (HFrEF). The Ventura device includes a nitinol hourglass-shaped frame that anchors to the patient’s fossa ovalis in a way that prevents migration or embolization. It is implanted via an interventional procedure with fluoroscopy and echocardiography guidance.
The Venture device was the focus of the very first late-breaking clinical trial presented at ACC.24, the annual meeting of the American College of Cardiology. Gregg Stone, MD, a professor of cardiology and population health sciences at Icahn School of Medicine at Mount Sinai, presented data from RELIEVE-HF, a randomized trial designed to evaluate the safety and effectiveness of treating heart failure patients with V-Wave’s new technology. RELIEVE-HF included more than 500 patients randomized to either undergo treatment with the device or undergo a placebo procedure.
After a median follow-up period of 22 months, Stone reported that treatment with the device was safe for all patients and provided significant for patients presenting with HFrEF. Patients with heart failure with preserved ejection fraction, however, did not benefit from treatment.
Stone spoke with Cardiovascular Business at ACC.24 about the trial, emphasizing that its impact on patients with HFrEF was “very, very positive.”
The Venture Interatrial Shunt System has not yet gained full approval from the U.S. Food and Drug Administration (FDA). It did receive the FDA’s breakthrough device designation in 2019, however, suggesting the agency has been helping V-Wave through the approval process.